PharmAust’s anti-cancer drug passed an important test on the way to human trials

'Wanna fight? Fight me." Picture: Getty Images
Oncology drug developer PharmAust (ASX: PAA) says its anti-cancer drug monepantel can deliver a “double kick” to human cancer cells.
Importantly, human cancer cell tests on human cancer cell lines showed it can do so with a reduced tendency to kill off healthy cells compared to traditional cancer-fighting methods.
PharmAust jumped on de-worming drug monepantel in April last year after winning a licence to use elements of it from its owner, Elanco.
The licence was to use it in clinical trials in dogs.
Before he jumped ship to medical cannabis biotech Zelda, PharmAust’s CEO Richard Hopkins said dogs trials made the process cheaper and faster to get a human drug out the door.
He said a a canine drug could get through the Phase 3 trials for $2 million to $5 million in three to five years.
The latest trial by researchers from the Olivia Newton-John Cancer Research Institute (ONJCRI) was on cancer cell lines that were representative of human cells PharmAust will treat in upcoming Phase II trials.
The ONJCRI team found that in both humans and dogs, “monepantel is metabolised to monepantel sulfone”. That metabolite, they found, remained in the body for some time.
- Bookmark this link for small cap breaking news
- Discuss small cap news in our Facebook group
- Follow us on Facebook or Twitter
- Subscribe to our daily newsletter
Importantly, it appeared “to have the same, targeted cytotoxic effect upon cancer cells and the same non-toxic effect upon non-cancer cells as monepantel.”
Result?
“Monepantel and its metabolite are predicted to provide and enduring and specific effect through a ‘double kick’ to cancer cells while minimally affecting normal cells in the body.”
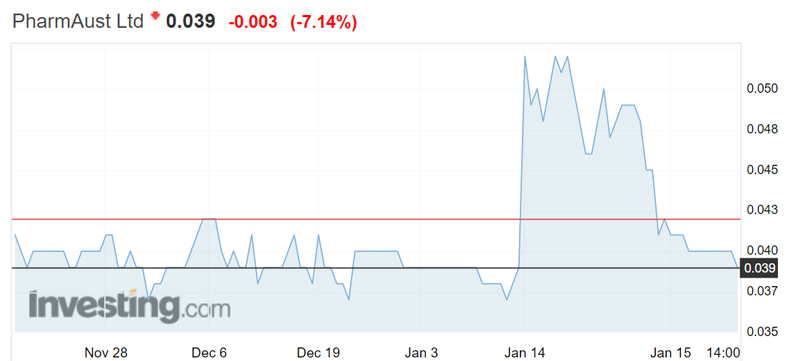
PharmAust shares rose to a three-month high of 0.052 yesterday before dropping back to 0.039 this afternoon.
The result puts PharmAust in the position to fine-tune the drug so that it may be taken “only a few times a week” rather than daily if further testing proves it effective.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








