Ongoing Phase 2 results from the study of Monepantel to treat canines with B cell lymphoma have been encouraging. Now PharmAust will expedite the study by taking it to the US.
PharmAust (ASX:PAA) has recruited the first pet dog with B cell lymphoma in its US canine Phase 2 trial assessing the efficacy of its lead asset, Monepantel (MPL).
The trial will treat 10 dogs as per FDA guidelines, and will be conducted by Dr Meighan Daly DeHart and her team at the Thrive Pet Healthcare and Heart of Texas (HoT) Veterinary Specialty Center in the US.
Within the last week, the first dog has passed a physical exam and certain standardised staging tests, and was sent home to commence treatment with MPL tablets.
The dog will be required to return for appraisal on days 14 and 28 at the Heart of Texas with Dr Daly DeHart.
“It is very exciting to start the first enrollment in the US,” said Principal Investigator, Dr Kim Agnew.
“During a recent visit to the study site, I found the team at Heart of Texas provide very compassionate patient-focused treatment, and I know the study enrolments are in exceptional hands.
“The clinic experiences a high oncological case load, so we are hopeful the enrolment rate will be expedited,” he added.
Expediting Phase 2 study
MPL has demonstrated effective anti-cancer activity and minimal side effects, with ongoing results so far showing encouraging results.
In the Phase 2 trials conducted in Australia to date, 29 pet dogs have been treated using MPL monotherapy.
Of the 16 pet dogs with optimum blood levels, 13 have achieved stable target lesions. This includes one dog with a partial response (60% regression).
Nine of the 16 dogs with optimum blood levels have achieved stable disease by RECIST (Response Evaluation Criteria in Solid Tumours). Side effects were minimal or not detected.
PharmAust requires greater than or equal to 18 dogs with a clinical benefit out of 46 dogs to meet its statistical endpoint.
Post-trial, some vets and the respective pet owners have elected to continue the MPL treatment and, sometimes, in combination with current standard of care, prednisolone.
The combination of MPL with prednisolone – which has provided average extension of survival to these pet dogs of 16-24 weeks – was shown to more than double the life expectancy of the dogs.
Following these encouraging results in Australia, and in preparation for Phase 3 registration trials, veterinary trial centres have been set up in New Zealand and the United States.
The US expansion announced today builds on the trial sites in Australia and New Zealand, and was designed to accelerate the enrolment of case numbers required to enable PharmAust to close out the Phase 2 study as quickly as possible.
The table below outlines the status of all dogs in the study:
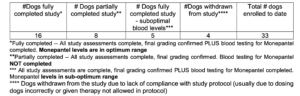
Please note the explanation of the definitions used in the table. Table: Supplied
Commercial opportunities
There is a potentially large commercial opportunity for MPL in canine cancer.
The drug is already approved for veterinary use for a different indication in food-chain animals, and Pharmaust is now looking to repurpose MPL as a safe and effective cancer treatment without the associated side effects of chemotherapy.
Dr Agnew stated, “I have talked to a number of large pharma companies about the opportunities for monepantel, and the feeling I get is that these pharma companies support the middle ground that monepantel provides, between that of prednisolone and chemotherapy.”
PharmAust is currently in confidential exploratory discussions with a leading global pharmaceutical company to co-develop and commercialise MPL for the treatment of veterinary cancers.
Typically, a licensing deal involves an upfront cash payment, plus remuneration of costs spent on developing the drug which would now be around $20m—$25m, as well as a 10-12% royalty on all sales of the drug.
Such a deal would mark a significant commercial outcome for PharmAust, and see it more than fully funded for all foreseeable future clinical trials.
Dialogue is continuing, and the company will also seek input from these potential licensing partners in preparation for the Phase 3 registration trial.
“The commercial target is to develop and partner a product that supersedes the current standard of care (prednisolone),” said Dr. Agnew.
“This is to provide a canine lymphoma treatment option that can be administered daily by the owner and enabling excellent quality of life for the dog during treatment.”
This article was developed in collaboration with PharmAust, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
You might be interested in












