Paradigm hits record high after osteoarthritis patients report reduced knee pain
Breakthrough ... new arthritis drug could be on the way. Picture: Getty
Osteoarthritis patients treated with Paradigm Biopharma’s repurposed drug are reporting significant reduction in knee pain ahead of a formal release of trial results expected before Christmas.
The news pushed the shares up 15 per cent this morning to a record high of $1.10. The stock (ASX:PAR) has more than tripled from around 30c in April.
Paradigm has staked its livelihood on the efficacy of a drug called Pentosan Polysulfate Sodium, or PPS, which has been used for half a century to treat conditions such as blood clots and bladder pain.
Paradigm is using an injectable form of the drug to treat patients with significant osteoarthritis.
It is available via the Therapeutic Goods Administration Special Access Scheme, under which doctors can prescribe unapproved treatments for patients if they fit specific criteria. It is also being evaluated in an ongoing Phase 2b clinical trial.
Clinical trials are generally divided into three phases. Phase 1 focuses on safety, Phase 2 tests for effectiveness and Phase 3 examines whether the new drug is an improvement on existing treatment. Sometimes trials are further divided into parts A and B, where a B stage is generally more rigorous.
Today Paradigm told investors that 145 patients treated under the scheme had reported an average 51.2 per cent reduction in knee pain. Final results from the clinical trial are due before Christmas.
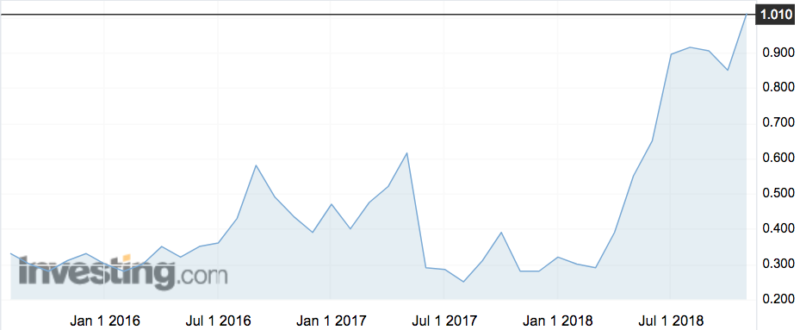
The average knee pain reduction of over 50pc is considerably higher than the “typical 15pc pain reductions scores reported for opioid treatments”, the company says.
Over the 145 patients treated, 86.8pc said they had felt a reduction in joint pain and 91pc said knee function had improved.
Paul Rennie, Paradigm chief, said the data provided real world evidence that could be used in conjunction with the anticipated positive clinical trial data to get the drug onto market shelves.
“Given these patients have a very similar treatment regimen to subjects being treated under the current Phase 2b osteoarthritis clinical trial and these patients have failed current therapies to treat the condition, we feel particularly confident regarding a positive clinical trial outcome,” he said.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








