Osteopore hits record revenue in Q3 and achieves 4 consecutive quarters of revenue growth, ticks boxes for future growth

Pic: Getty Images
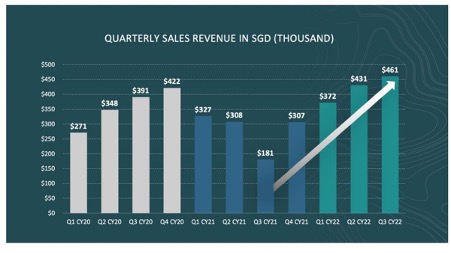
Osteopore achieves record revenue in Q3 CY22 as demand increases for its implants.
Regenerative bone and tissue implant specialist Osteopore (ASX:OSX) has achieved record revenue of S$460,684 ($482,890) for Q3 CY22.
Revenue increased 6.6% over Q2 CY22, resulting in the fourth consecutive quarter of revenue growth, and a 164% increase over the previous year’s revenue for the corresponding period of Q3 CY21.
The strong result was led by increasing sales from Vietnam and Singapore, along with consistent volume of implants for rhinoplasty (nose surgery) applications within Korea.
Increasing numbers of patients are going to hospital to seek treatment and surgeons are getting familiar with Osteopore’s technology, which has led to an increase in neurosurgery cases and demand for Osteopore’s implants.
The company said it was particularly witnessing strong demand from Vietnam, where over 40% of implants delivered in July were used in surgeries.
“This required another large order to be shipped during the quarter to meet demand,” Osteopore said in its update.
Use of its implants in rhinoplasty continues to grow, with Osteopore increasingly gaining exposure at exhibitions around Asia, particularly in South Korea.
Grant to develop ‘holy grail’ of bone regeneration
In late September, Osteopore secured a US$225,000 non-dilutive grant from the Government of Chile Scientific and Technological Development Support Fund.
The grant will be used to develop a novel 3D-printable implant that accelerates bone regeneration.
Osteopore’s implants currently enable the natural stages of bone healing and it claims they are superior to other traditional bone regeneration procedures.
By incorporating materials and compounds that speed up bone growth, a patients’ recovery could be accelerated.
If successful, this would be a world first breakthrough that places Osteopore at the forefront of technology development and considered the ‘holy grail’ of bone regeneration.
Distribution agreement secured for US Federal Healthcare Facilities
Osteopore’s US based distributor Bioplate Inc secured a non-exclusive distribution agreement with MellingMedical LLC to promote and sell Osteopore products within healthcare facilities owned or operated by the US federal government.
The initial focus will be supplying products to Veterans’ Affairs and Department of Defense facilities that have neuro and trauma surgery capabilities.
MellingMedical has access to more than 165 Veterans’ Affairs (VA) Medical Centres, 300 VA Outpatient Clinics, all seven Consolidated Mail Outpatient Pharmacies, and 95 Department of Defense Medical Facilities.
Bioplate and Osteopore are now working with MellingMedical to apply for listing in the Federal Supplies Schedule (FSS).
Upon successful listing confirmation, Osteopore products will be available to all federal healthcare facilities.
Distribution agreement signed to enter the Chinese market
Osteopore signed a distribution agreement with Kontour (Xi’an) Medical Technology (Kontour), to market and sell Osteopore products within China.
Kontour is a leading manufacturer and distributor of surgical supplies across China, and is listed on the Shanghai Stock Exchange with an estimated market value of ¥3.38bn ($711m).
Under the terms of the exclusive four-year agreement, Kontour and Osteopore will conduct several clinical trials to obtain the required data to achieve National Medical Products Administration (NMPA) approval.
If successful, NMPA approval will allow Osteopore products to be marketed and sold across China.
Kontour has significant experience in the Chinese regulatory process, and anticipates NMPA approval will take an estimated 24 months.
The focus will be gaining clearance for Osteopore’s craniofacial products. Kontour has agreed to make every reasonable endeavour to purchase a minimum US$500k of Osteopore products in the first year after NMPA clearance, and US$1 million in the second year.
Products added to the Australian Prostheses List
All Osteopore’s products currently listed for use under the Australian Register of Therapeutic Goods (ARTG) will be formally included into the Australian Prostheses List on November 25.
The list identifies implantable devices eligible for reimbursement from all private health insurance funds, with Osteopore products undergoing a rigorous application and review process to be included.
Being incorporated into the list ensures all Australian citizens with private healthcare coverage can be reimbursed for Osteopore products, with surgeons being able to choose the best available prostheses for privately insured patients as covered by all the individual healthcare insurance funds.
Being included on the list is expected to remove barriers to the recommendation of Osteopore products to suitable patients as the implants could potentially reduce costs to patients.
Commercial collaboration with Singular Health
Following evaluation of the recently completed 3Dicom VSP module for virtual reality, whereby Osteopore and Singular staff remotely collaborated on cranial scans in real-time, Osteopore agreed to four enterprise licences of the 3Dicom R&D and VSP software.
The licences will see Osteopore branding added to the 3Dicom User Portal for surgeons to visualise its products in virtual reality along with their own scans for training and marketing purposes.
The synergies between the companies have already led to joint presentations and conversations by Osteopore and Singular Health to third parties in the USA, Australia, and Europe.
Delivering on growth strategies
Osteopore executive chairman Mark Leong said the company continues to deliver on its growth strategy put in place earlier this year, and as a direct result revenue is beginning to scale.
“During the quarter, we made encouraging progress to re-ignite sales in the US market by gaining exposure to healthcare facilities owned or operated by the federal government,” he said.
“We also deepened our penetration into the Australian market and signed a distribution agreement to market and sell Osteopore products within the People’s Republic of China.
“Importantly, our research and development programs continue to produce results which could present significant commercial opportunities in the future.”
This article was developed in collaboration with Osteopore, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








