Noxopharm spruiks anti-cancer clinical data; shares gain 5pc

New clinical data from cancer warrior Noxopharm suggests its NOX66 drug has an effect on the quality of life in patients with late-stage, spreading cancer.
Noxopharm (ASX:NOX) told investors today that it had convened a meeting with a US medical advisory board to discuss the latest data from a trial which uses NOX66 — a variation of the experimental anti-cancer drug idronoxil — to supercharge the effect of radiotherapy in patients undergoing palliative treatment.
The first clinical study for the program involves 24 men with late-stage, metastatic prostate cancer receiving palliative radiotherapy.
They’re broken into groups of four men receiving different daily dosages of NOX66, ranging from 400mg to 1200mg, and then scanned at six-week intervals to determine tumour response.
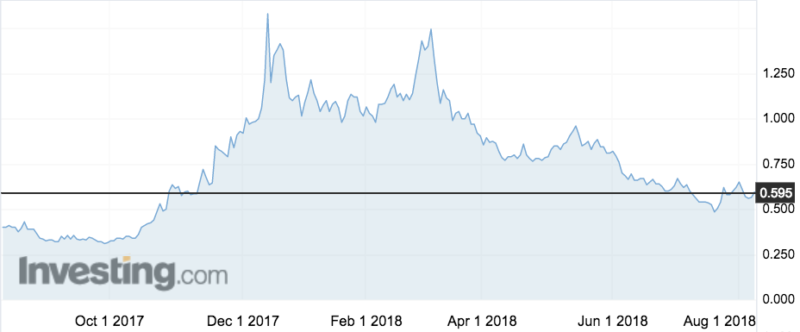
NOX66 has already shown signs that it works – the company’s stocks spiked in November last year upon the release of promising early clinical data in a different study. They’ve declined since, but were up 5 per cent to 59.5c on this morning’s news.
In palliative radiotherapy, patients receive a low dosage of radiation directed at one or two tumours to relieve pain symptoms.
The trial (known as “Direct and Abscopal Response to Radiotherapy program or DARRT) is studying whether a combination of the treatment and NOX66 will improve tumour response, resulting in improved survival and quality of life.
“Abscopal” refers to the ability of localised radiation treatment to trigger anti-tumour effects in other areas.
The trial is judged on two outcomes: direct, improved response in the targeted, irradiated tumours; and “abscopal” response, improved response in non-targeted tumours.
The abscopal response is the “ultimate goal”, says the company, particularly in patients with metastatic cancer where it is complete and permanent.
Improved palliative outcomes
Noxopharm says the program offers the potential to achieve improved palliative outcomes “without the rigours of chemotherapy, with minimal discomfort to the patient and to be highly cost-effective”.
They launched the DARRT program after early clinical results found two patients who received NOX66 on a compassionate use basis both showed an abscopal response.
Noxopharm CEO Graham Kelly developed NOX66 before he was chief of Novogen (ASX:NRT). Stricken with late-stage prostate cancer and facing death, he tried the drug himself – and it worked, leaving him free to continue leading the drug’s developed with Noxopharm at 72 years of age.
He said the Phase 1 safety and tolerability study was demonstrating exciting signs.
“So far we’ve treated seven patients with the DARRT program and four of those seven have shown an abscopal response varying from partial to complete,” he said. “We’re now at the dose we consider our therapeutic dose, with the first patient at that dose showing early signs of a strong anti-cancer effect and an abscopal response.
- Bookmark this link for small cap breaking news
- Discuss small cap news in our Facebook group
- Follow us on Facebook or Twitter
- Subscribe to our daily newsletter
“We have good reason to believe we are on track to achieve the first evidence of this being possible in a reasonable proportion of prostate cancer patients.”
The Philadelphia meeting comprised 10 medical and radiation oncologists from major US hospitals and recommended that DARRT be extended into pancreatic cancer, head and neck cancer and primary brain cancer.
The company plans to review the opportunities in these indications, and says it aims to commence a multi-national Phase 2 study of NOX66 in mid-2019.
Patient recruitment for the Phase 1 study is ongoing.
Noxopharm has been contacted for comment.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








