With no HIV cure in sight, Biotron focuses on China Hep C patients

Pic: Luis Alvarez / DigitalVision via Getty Images
You could be forgiven for thinking that a cure for HIV had been found when, on World AIDS Day, scientists reported a French drug-repurposing trial showed a ‘drastic and persistent decrease’ in the cell reservoirs of an HIV-infected patient.
HIV (Human Immunodeficiency Virus) is a virus that attacks the body, weakening it until it reaches a compromised state where it is threatened by infections as varied as respiratory illness and hepatitis C.
Globally, 70 million people live with HIV and another 71 million with Hepatitis C, the United Nations estimates.
The report spawned headlines such as ‘HIV CURE!’ and ‘Evidence that a cancer drug may be able to eradicate HIV-infected cells in humans’.
However, there was more than a little hyperbole in the headlines.
“This is the first case of such a drastic decrease of the HIV reservoir, and we must remain careful, especially because this is only one case,” said Professor Jean-Philippe Spano, head of the medical oncology department at Pitie-Salpetriere Hospital AP-HP in Paris.
“We have published details of another case where there was no decrease of the HIV reservoir.”
Potential toxicities of the treatment were unknown and there was much more work to be done, the professor said.
Positive results
As exaggerated as they were, the headlines may have given hope to ASX-listed biotech Biotron, which has for many years been developing a small-molecule therapy, BIT225, to treat viral infections including HIV-1 and hepatitis C.
Now in phase 2 of clinical trials, Biotron has been using a “similar but slightly different” approach to the French trial by targeting the long-lived macrophages (or reservoirs) in the body to rid the body’s of the HIV-1 cells.
The trial has shown positive results in treating hepatitis C when used in conjunction with existing low-cost hepatitis C drugs interferon and ribavirin.
“We were able to mimic this earlier in the year in mice with a humanised immune system, said Biotron chief Dr Michelle Miller.
“These mice can be infected with HIV, treated with drugs to reduce HIV levels, and then we took away all drugs.
“The addition of BIT225 to ART [anti-retrovital treatment] in these mice resulted in a faster decrease of virus levels to undetectable, and there was a delay in rebound of virus once all drugs were stopped.
“This is a very encouraging indication that our drug is targeting this key reservoir.”
There is still lot of research to be done in the HIV reservoir in other sanctuary organs such as the gut and brain, where most of the HIV cells are said to reside. That’s the reason HIV can keep re-emerging after repeated treatment.
Focus on Hepatitis C
Biotron is continuing to work on the HIV problem, but focusing commercial efforts now on hepatitis C.
Biotron is seeking partnerships in Shanghai, China, starting with its hepatitis C product — a move targeted at the high co-infection rate of hepatitis C and its related immunoviruses, in a market with ten times more patients than the United States.
In China, the current treatment for hepatitis C using the combination of interferon and ribavirin costs 46,000 RMB ($9,200) and requires a treatment cycle of 48 weeks.
Biotron’s main competitor is the direct-acting antiviral drug Harvoni, which only takes eight weeks of treatment — but costs 600,000 RMB ($120,000).
Harvoni also requires diagnostic treatment to monitor for prior co-infection and ongoing re-activation of Hepatitis B and other immunosuppressive conditions.
Biotron’s BIT225 however has shown that by combining the standard low-cost interferon/ribavirin treatment cycle — starting with an additional four to 12 weeks treatment of BIT225 — can improve the clearance rate of the most common type of Hepatitis C genotype in Chinese patients.
Using the lower cost advantage and the benefit of BIT225’s minimal reactivation in co-infected patients compared to its other direct-acting antiviral treatment competitors, Biotron is seeking development partners in China where there are some 10 million co-infected patients.
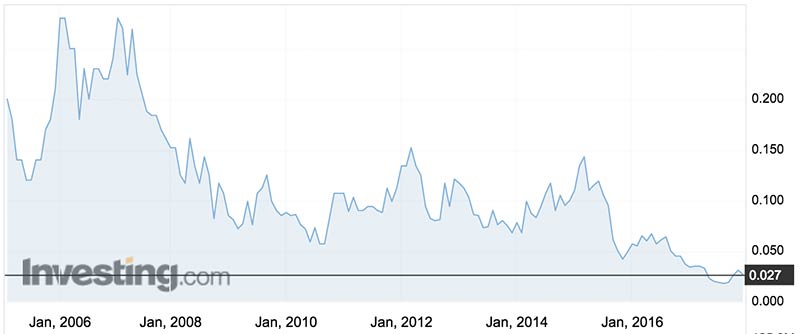
Historic lows
Biotron’s share price is at historic lows of about 2.7c — a long way from the heights of 28c they fetched in 2006 and 2007.
The company burned about $1.2 million last quarter, leaving $830,000 in the kitty. — although a $1.6 million R&D rebate was welcomed in November.
Estimated spending this quarter was due to clock in at $689,000 — though Dr Miller has assured investors that “Biotron is fully funded for its current activities, which include the current Phase 2 HIV-1 trial”.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








