No crap: Immuron is one step closer to an anti-gastro pill

Pic: Charnchai / iStock / Getty Images Plus via Getty Images
No need to fear the dodgy fish curry served up by your cut rate airline, as Aussie biotech Immuron is moving its anti-gastro pill into pre-clinical trials.
None other than the US Department of Defense (DOD) is funding the $55 million company’s study to find a way to kick Delhi belly in the gut before it starts — after the last of three studies suggests it might work.
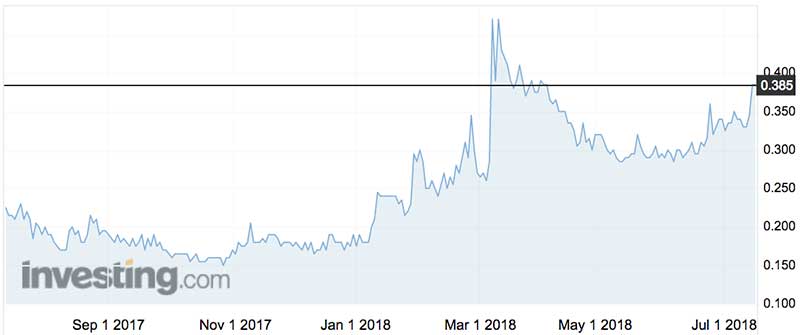
Immuron shares gained 12 per cent to 38.5c in early Monday trade.
In January, Immuron (ASX:IMC) released a study of its Travelan tablets by the DOD which showed its was effective in preventing travellers diarrhoea in 180 strains of campylobacter.
On Monday, studies by the US Naval Medical Research Center and Walter Reed Army Institute of Research found that it also worked for Enterotoxigenic Escherichia coli (ETEC) and Shigella, a nasty and virulent version of gastroenteritis commonly picked up in developing countries.
Gastroenteritis, also known as ‘Montezuma’s revenge’, is one of the world’s most common infections — as weak-bellied travellers to countries like India will attest.
It strikes within 12 to 72 hours and can last weeks. The infection caused the stomach and intestinal tract become inflamed which creates diarrhoea, nausea and vomiting, and abdominal pain.
There are about 1.5 billion cases of diarrhoea every year, according to the US National Center for Biotechnology Information.
It’s a very serious health problem in some parts of the world — resulting in about 2.2 million deaths, mostly of children under five years old in developing countries.
“[Immuron’s drug] Travelan has been designed to target selected surface antigens from the most common strains of Enterotoxigenic E. coli,” said Immuron CEO Dr Jerry Kanellos.
“The work completed at the US Armed Forces Research Institute of Medical Sciences, US Naval Medical Research Center and the Walter Reed Army Institute of Research has highlighted that in laboratory testing Travelan was effective across all strains and species of enteropathogenic bacteria tested.”
>> Read Tim Boreham’s recent profile of Immuron here
The company says preventing outbreaks of gastro and specifically Shigella is a “high priority” for the US army.
The company launches pre-clinical trials at the end of this month at the Department of Enteric Diseases of AFRIMS, an overseas laboratory of the Walter Reed Army Institute of Research, in Bangkok.
They want to see just how effective the drug is at stopping Shigella before it explodes.
The DOD is paying for the trials and interim Dr Kanellos told Stockhead if it goes well, they’re keen to fund human trials after that.
- Subscribe to our daily newsletter
- Bookmark this link for small cap news
- Join our small cap Facebook group
- Follow us on Facebook or Twitter
The company sells itself as a gut immune and microbiome clinical development company, but its gastro cure-cum-preventative pill Travelan is rapidly becoming what it’s known for.
Its sole source of revenue is from Travelan sales. The pill launched in 2004 in Australia, but an alliance with a US travel clinic has sent sales rocketing.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.