Neurotech moves to show economic advantage of autism treatment as Phase II/III trial results due soon

Autism is a major rising cost to the NDIS in Australia. Pic via Getty images.
- Neurotech views lead drug NTI164 as a promising, cost-effective and long-term treatment for ASD
- Health economic consultancy appointed to evaluate economic benefits of treating ASD
- Recruitment finalised for Phase II/III trial of NTI164 with results forecast for Q1 CY24
Special Report: Neurotech International is working to evaluate the economic benefits of treating autism spectrum disorder (ASD) patients in Australia as studies indicate a significant cost savings to the National Disability Insurance Scheme (NDIS) from early interventions.
Clinical-stage biopharmaceutical development company Neurotech International (ASX:NTI) says during Q2 FY24 it appointed a renowned health economic consultancy to identify and quantify the value proposition of treating patients with ASD in Australia.
NTI says past studies have indicated significant cost savings to the NDIS from early autism interventions, primarily through occupational therapies due to a lack of effective drug treatments.
However, NTI sees promising potential in its lead drug therapy NTI164 as a cost-effective and safe long-term therapy for ASD.
The company’s optimism is based on the positive outcomes from the Phase I/II clinical trials of NTI164 over 52 weeks, along with its pending Phase II/III clinical trial results forecast for Q1 CY24.
NTI is focused predominately on paediatric neurological disorders, where there is persistent neuroinflammation.
Analysis to aid future NTI164 pricing
The analysis is expected to greatly assist NTI in future discussions with government and private payers relating to the appropriate pricing and reimbursement for its proprietary broad spectrum cannabinoid therapy NTI164.
The company says preliminary cost-effectiveness (CE) and pricing analyses for NTI164 will be modelled to provide price estimates sufficient for determining the feasibility of Pharmaceutical Benefits Scheme (PBS) reimbursement for NTI164.
The PBS schedule lists all the medicines available to be dispensed to patients at a Federal Government-subsidised price.
NTI says additionally the feasibility assessment will review the strength, quality and applicability of the clinical evidence base that will be available at the time of a PBAC submission.
Under a PBAC submission, NTI will be required to provide an economic evaluation of NTI164, including its proposed cost-effectiveness.
ASD major cost to NDIS
NDIS statistics reveal that 35%, or 214,880 participants, were diagnosed with autism, a figure that rose to 223,650 as of September 30, 2023.
Moreover, 78% of those with autism are under 18, with the 7–14-year-old group accounting for 52% of these participants. Additionally, 70% of these individuals are male.
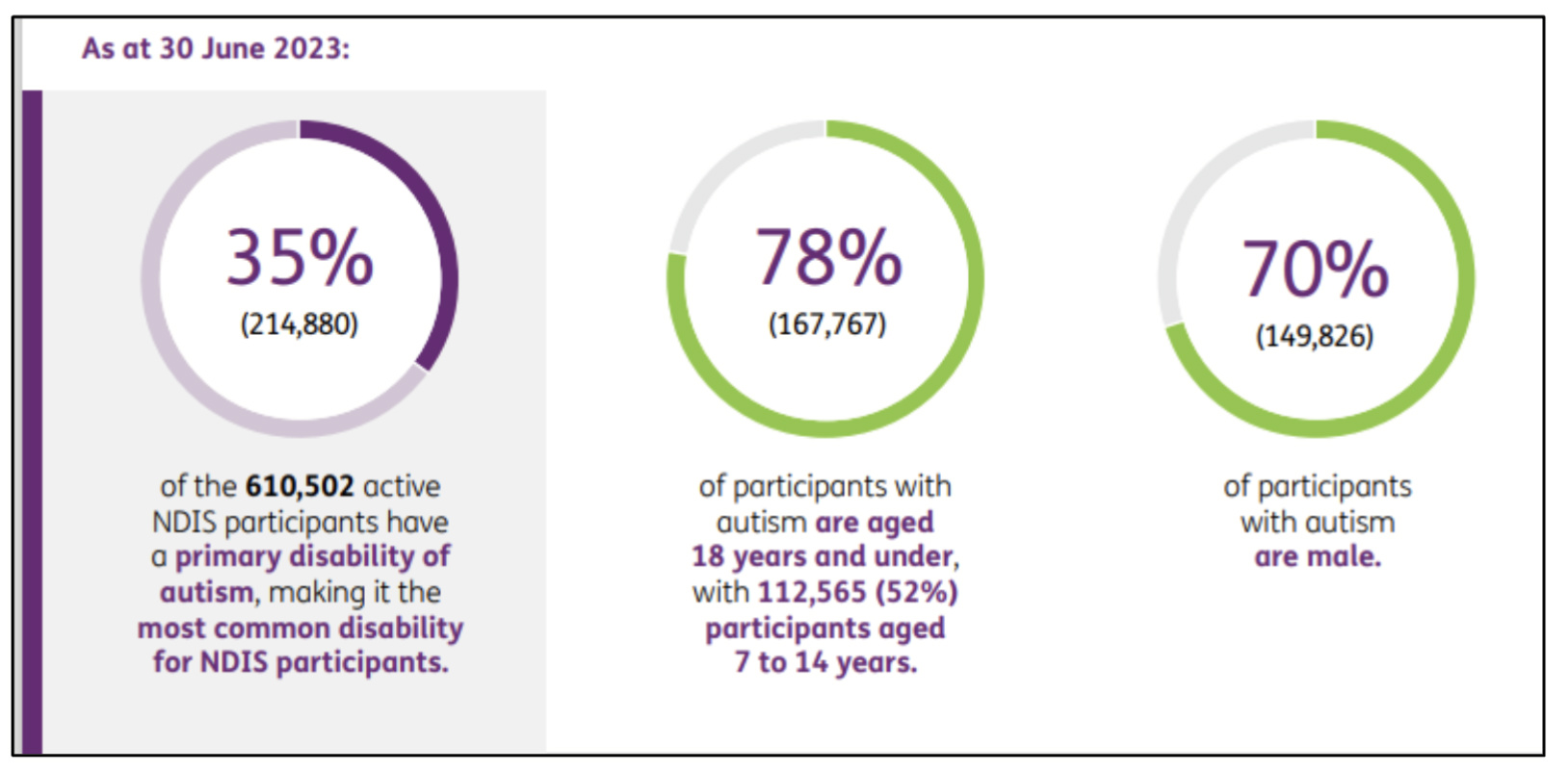
Source: NDIS data
NTI says in FY23, the NDIS allocated $6.73 billion to support individuals with autism, marking a substantial 28% rise from the previous year’s $5.27 billion.
Funding covered payments to both people with autism and their service providers with the average support payment for a participant with autism in FY23 being $33,800, up 7% from FY22.
Most of the funds (~$2.7 billion) was primarily spent on core support in daily activities.
NTI says with substantial increase in ASD-associated costs under the NDIS there is an urgent need for new enabling treatments like NTI164, shown to significantly improve adaptive behaviours and socialisation and improve the quality of life of child sufferers while reducing caregiver burden.
The prevalence ASD is estimated at one in 50 across the population, representing a 40-fold increase in the last 20 years.
Phase II/III recruitment complete
In December, NTI announced the completion of patient recruitment for its Phase II/III NTIASD2 clinical trial.
The trial has recruited 56 patients with Level 2 (requiring substantial support) and Level 3 (requiring very substantial support) autism.
All patients were enrolled at the Paediatric Neurology Unit at Monash Medical Centre, through the trial’s Principal Investigator Professor Michael Fahey, who is the head of the paediatric neurology unit and director of neurogenetics.
The Phase II/III randomised, double-blind, placebo-controlled clinical trial is evaluating the effectiveness and safety of NTI164 in patients with ASD compared to a placebo.
The study includes an eight-week treatment phase, an eight-week open-label maintenance phase and a two-week wash-out period.
NTI says participants wishing to extend NTI164 use beyond the study can do so for an extra 38 weeks, followed by a two-week down-titration phase at the end of this extension.
Other quarterly highlights and outlook
NTI says during Q2 FY24 it has achieved notable progress in advancing the application of NTI164 for various paediatric neurological disorders with a critical need for new, safe and effective treatments.
Looking for the rest of the financial year NTI expects several key developments including:
- Human Research Ethics Committee (HREC) approval for a Phase I/II clinical trial in paediatric spastic cerebral palsy, anticipated in Q3 FY24;
- The unveiling of results from the Phase I/II clinical trial for Rett Syndrome in Q3 FY24
- Outcomes from the Phase II/III ASD clinical trial, also expected in Q3 FY24;
- Pursuing orphan drug designations (ODDs) for NTI164 in PANDAS/PANS from regulatory authorities in the US and EU; and
- ODDs for NTI164 in Rett Syndrome with US and EU regulatory bodies.
NTI says it remains fully funded to complete these current clinical trials through to top-line results.
This article was developed in collaboration with Neurotech International, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








