Neurizon’s ALS drug shows safety and extended survival in open-label extension study

Neurizon reports positive results of its OLE study of NUZ-001 to treat ALS. Pic via Getty Images
- Neurizon’s open-label extension study of lead candidate NUZ-001 for amyotrophic lateral sclerosis reaches primary endpoint
- Study shows the drug at recommended phase II dose was safe and well-tolerated
- NUZ-001 significantly increased survival and reduced risk of death, slowing rate of functional decline
Special Report: Neurizon Therapeutics has reported positive topline results from an open-label extension (OLE) study of its lead candidate NUZ-001 for amyotrophic lateral sclerosis (ALS), the major form of motor neurone disease.
Clinical-stage biotech developing treatments for neurodegenerative diseases Neurizon Therapeutics (ASX:NUZ) said the study reached its primary endpoint, showing long-term treatment with NUZ-001 at the phase II dose was safe and well tolerated.
Importantly, preliminary efficacy findings demonstrated that treatment with NUZ compared to matched historical controls from the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) showed:
- Significant survival advantage
- Sustained slowing of global functional decline
- Reduced rate of respiratory decline
- Stable/downward biomarker trends
The PRO-ACT database is the largest publicly available dataset for ALS clinical trial data globally. Its size and size and breadth makes the database a critical external control resource for ALS drug development.
Neurizon said the OLE findings support the potential of NUZ-001 as a disease-modifying therapy for ALS and justify progression into late-stage clinical development.
About the study
The OLE study has been conducted at two clinical sites in Australia including Calvary Health Care Bethlehem, led by Associate Professor Susan Mathers, and Macquarie University, led by Professor Dominic Rowe.
It examined safety, tolerability and preliminary efficacy study in 10 patients who completed Neurizon’s phase I MEND study.
Patients were treated daily at the recommended phase II with 10 mg/kg of NUZ-001 for 12 months, which was safe and well-tolerated with no treatment-related deaths observed.
Eight patients completed the study, while two died due to respiratory failure and pneumonia.
Throughout the study, a total of 25 treatment-emergent adverse events (AEs) were reported, of which only three were considered possibly treatment-related – mild to moderate dry mouth at night, increased hair growth, and elevated liver enzymes.
Neurizon said there were no treatment-related severe AEs and no discontinuations due to an AE.
Since the phase I MEND study started in October 2022 five initial patients enrolled have now been on daily treatment of NUZ-001 for more than two and a half years under the Therapeutic Goods Administration (TGA) Special Access Scheme.
Key results of trial
In the OLE study, disease progression was tracked using the US Food and Drug Administration (FDA) accepted primary endpoint for ALS trials – the ALS Functional Rating Scale-Revised (ALSFRS-R).
Patients showed a similar rate of functional decline to those in the earlier phase I MEND study, suggesting a stable and lasting treatment effect with NUZ-001.
Slow Vital Capacity (SVC) decline is a validated marker of disease progression and survival in ALS.
In the OLE study, the mean vital capacity per cent predicted (VC PP) at baseline 72.4% and this declined gradually to 62.3% after 12 months.
Neurizon said this decline was not significantly different from results seen in the earlier phase I MEND study, indicating a stable and durable treatment effect with long-term NUZ-001 therapy.
Other measures, including quality of life scores (ALSSQOL-R) and Edinburgh Cognitive and Behavioural ALS Screen (ECAS), also showed stable results over the long term.
Biomarker data supported these findings, with plasma NfL (neurofilament light) levels staying mostly stable over 12 months and urinary p75ECD falling by about 17%.
Together, these results point to a lasting treatment effect and support the potential of NUZ-001 as a disease-modifying therapy for ALS.
Slower rate of decline to PRO-ACT database
A comparative analysis against the PRO-ACT database was conducted for the entire treatment period including the phase I MEND and OLE studies, reflecting continuous NUZ-001 administration.
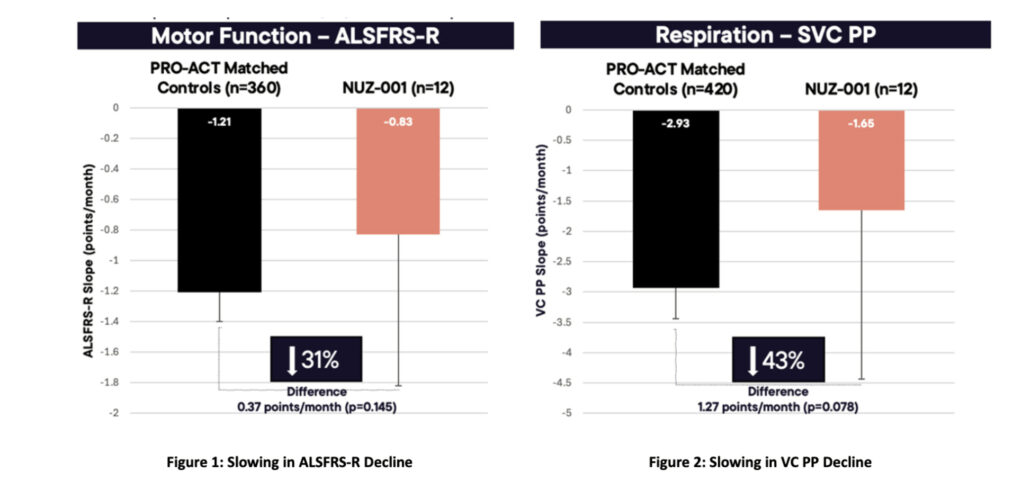
Results show patients treated with NUZ-001 also experienced a slower decline in ALSFRS-R compared with untreated matched historical controls from the PRO-ACT database.
Specifically, NUZ-001 treatment was associated with a 0.37 points/month slower decline, corresponding to a 31% reduction in disease progression.
Similarly, the estimated decline in vital capacity percent predicted (VC PP) was 43% slower.
NUZ-001 demonstrates survival signals in study results
As of August 15, 2025, half of the 12 patients in the initial phase I MEND study are still alive. Patients have been on treatment for between 11 and 34 months, with the average being about 29 months.
Even using the most cautious analysis, the data show a clear survival benefit. The treatment reduced the risk of death by around 76% compared with similar patients who did not receive NUZ-001, and an extended median survival of ~16 months.
Next steps – the HEALEY ALS Platform Trial
Encouraged by the results, Neurizon is set to advance NUZ-001 to a pivotal phase 2/3 HEALEY ALS Platform Trial in Q4 CY25, pending FDA clinical hold clearance.
The company aims to confirm the observed benefits in a larger, placebo-controlled setting in the HEALEY trial, which is a significant ongoing program in the US aimed at accelerating ALS treatment development.
The OLE findings reinforce the company’s confidence in its clinical development strategy for NUZ-001 and validate the selected dosing regimen for the HEALEY ALS Platform Trial.
“NUZ-001 has been a well-tolerated therapy, and we hope that the future phase 2/3 study will confirm its potential to benefit the wider ALS community,” said Mathers.
Dr Michael Thurn, Neurizon’s managing director and CEO, thanked those involved in the study and said that for too long ALS patients and their families have faced a devastating disease with very few treatment options, and the field has struggled to find truly viable new options.
“To see sustained functional and respiratory benefits, a clear survival advantage, and supportive biomarker trends after nearly three years of treatment gives us additional
confidence as we finalise preparations for entry into the HEALEY ALS Platform Trial,” he said.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








