‘Moving ahead with vigour’: Rhythm raises $3.5m for 2025 cancer test launch

Pic: Getty Images
- Rhythm Biosciences’ new CEO Dr David Atkins is on a mission to improve early diagnosis of bowel cancer
- Company raises $3.5 million in a placement supported by several new institutional, professional and sophisticated investors
- Second generation Colostat multiplex assay kit set to be launched commercially in 2025
Six months into the job and CEO of Rhythm Biosciences (ASX:RHY) Dr David Atkins believes the company is getting closer to significantly impact the early diagnosis of bowel cancer and, in turn, saving lives.
The scientist and healthcare leader has held roles across the pharmaceutical, medical device and diagnostics sectors globally, including with Congenica, Gene Shears, Johnson & Johnson and Danaher.

Dr Atkins took up the helm of Rhythm in May, ending a comprehensive and competitive global search for its CEO as the company looks to commercialise its lead asset, a bowel cancer screening blood test called Colostat.
“I’ve been in and around oncology for over 30 years and while there has been great progress in treatment, the fundamental problem for cancer is still early detection,” he said.
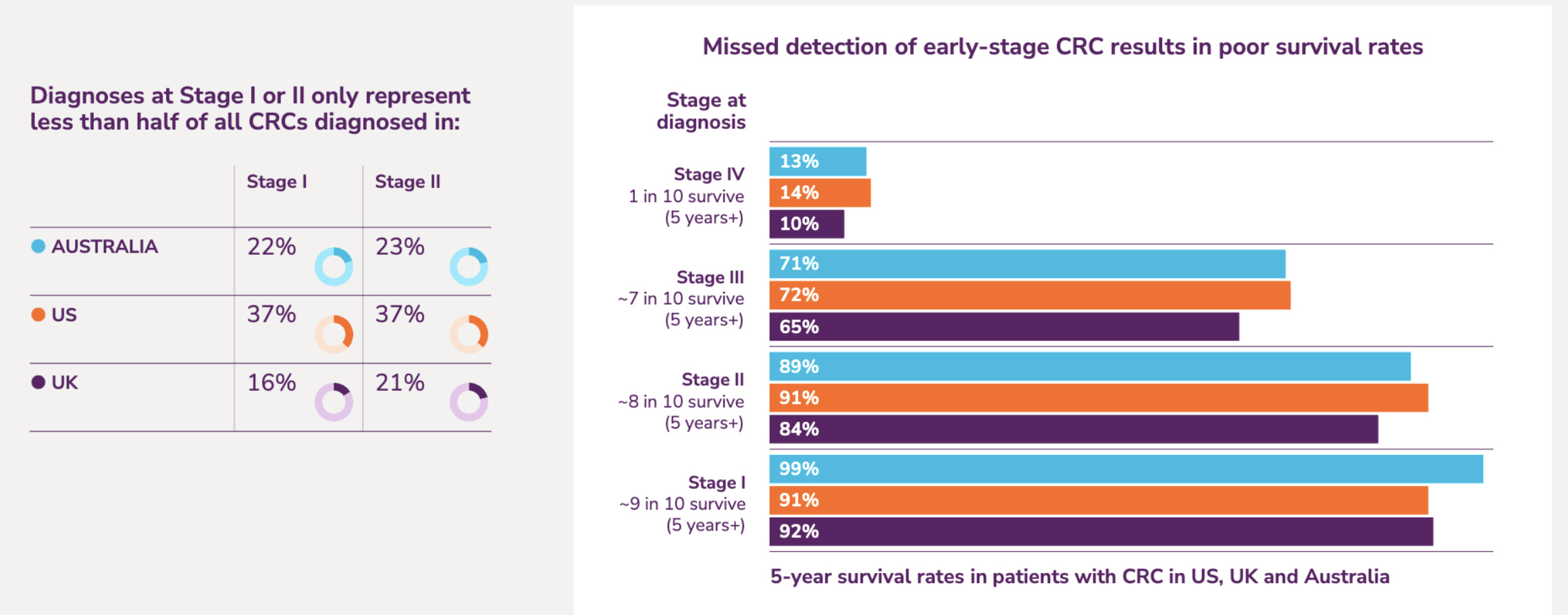
Bowel cancer remains a disease that gets diagnosed late, he noted, but if detected earlier the outcome for patients would be markedly better.
“What I would love to do with the Rhythm team and our supporters is produce products which have a material impact on patients and families around the world. So we have a relatively simple purpose and I believe we can do it,” Atkins said.
“It gets me up every morning – that privilege to think if we can do our job properly there are people whose lives will be saved.”
Overcoming the ‘ick’ factor in diagnosing bowel cancer
Also known as colorectal cancer, bowel cancer is the third most common cancer worldwide.
The World Health Organization said bowel cancer accounts for ~10% of all cancer cases and is the second leading cause of cancer-related deaths globally.
According to Bowel Cancer Australia, although most newly diagnosed bowel cancer cases occur in people aged 50 years and over, around 1 in 9 (11%) Australians diagnosed with bowel cancer are under the age of 50.
The Australian government’s national bowel cancer screening program provides free stool tests for those aged between 45 to 74.
However, what Stockhead’s Tim Boreham has described as ‘the ick factor’ of collecting a stool sample means low compliance.
“In Australia, 57% of the tests sent out in Australia get thrown into the bin and of those 43% that are used, there are a lot are false positives,” Atkins said.
He noted that studies have shown people are more likely to take a blood test as a screen than a stool test when given the choice.
“The evidence is overwhelming that if there is a relatively low-cost effective blood test it will transform screening and significantly shift the number of cancer cases detected earlier,” he said.
$3.5m capital raise to drive commercialisation strategy
This week Rhythm announced it had received commitments to raise $3.5 million in a placement supported by several new institutional, professional and sophisticated investors.
The company will issue 35,000,000 new fully paid placement shares at 10 cents each together with two listed options, with an exercise price of 20 cents, for every three placement shares issued with an expiring date of March 31, 2026.
“The goal was to raise $3 million, and we ended up raising more than that, which was a tremendous endorsement by largely new investors and shareholders,” Atkins said.
Funds raised will continue development of the second generation of Colostat multiplex assay kits and to undertake further clinical studies on the test.
The funds will also be used to progress Rhythm’s R&D pipeline into blood tests to detect other cancers.
“Our most advanced program is a series of protein markers which can be detected in blood for lung cancer,” Atkins said.
“Behind that we have a program for stomach cancer, along with breast, pancreatic and ovarian.
“These funds will help us move along the program and broaden our offering.”
Moving ahead with vigour
Rhythm listed on the ASX in December 2017 with science developed by the CSIRO over several years.
“The scientists who had done that work left CSIRO and became founding members of Rhythm with a huge amount of work going into the science, which is a great asset for the company,” Atkins said.
The initial Colostat assay kit received the regulatory tick in Europe and New Zealand. A submission for approval with the Therapeutic Goods Administration in Australia was delayed, however, as the company worked to refine the test.
Atkins said feedback from customers and laboratories was that the kit needed simplification.
“The initial assay was a collection of five different test tubes which had to be run in order to complete the assay,” he said.
The second generation Colostat multiplex assay kits combine the five separate antibody-based assays that previously constituted the original Colostat assay into a single reaction for each patient blood sample.
“We took one step back to reconfigure the assay so it’s much simpler and more in line with what the laboratory industry uses,” Atkins said.
“We had to take a step back, but we believe in the medium to long term it was the right step.”
Rhythm is looking to launch the second generation of Colostat in 2025, with preliminary testing on 200 patient serum samples with the alpha-version of the prototype assay demonstrating superior analytical performance compared to the first-generation product.
“In my experience with early-stage businesses you tend to make a lot of progress and tend to take a corrective step backwards step before moving forward with renewed strength and vigour,” he said.
“Rhythm, with our refined Colostat, is now moving ahead with vigour.”

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








