Micro-X reaches key Head CT milestone with imaging trials to start
Micro-X’s first Head CT test bench is ready to enter hospital trials for stroke imaging. Pic: Getty Images.
- First of three Micro-X Head CT test benches completed and ready for installation at an Australian hospital to begin stroke imaging trials
- Pivotal study to run over six to nine months comparing Head CT images with conventional CT scans of stroke patients
- Medical device designed for use in standard ambulances and retrieval aircraft to determine stroke type
Special Report: Micro-X, a leader in cold cathode X-ray technology for health and security markets, has completed its first Head CT imaging test system for the Australian Stroke Alliance (ASA) to install in a hospital and start phase I imaging trials.
It’s a major milestone for Micro–X (ASX:MX1), which noted the ASA had submitted a request for ethics approval to start the trials. Meanwhile final site approvals and regulatory licences are in process ahead of delivery of the Head CT test bench to the first trial site.
Once delivered, the device will be integrated into hospital systems and staff trained on use of the test bench with two additional Head CT test benches being developed for further trials in two Australian hospitals.
The milestone sparks a $400,000 payment to Micro-X, representing one-third of its final development contract with the ASA.
The first phase of the imaging validation trial is a blind reader study, where the Micro-X Head CT test bench will be used to take CT scans of 90 patients already admitted to hospital.
Those images will be compared to images taken using the hospital’s conventional CT, to confirm diagnostic equivalency.
Following a successful first phase trial, a pivotal study will start, which will image suspected stroke patients to compare Head CT images with hospital stroke CT images.
Micro-X said the pivotal study would run over six to nine months to image patients presenting with ischaemic stroke and the five types of intracranial haemorrhages associated with haemorrhagic stroke.
Portable Head CT tailored for pre-hospital stroke care
In partnership with the ASA and with $8 million funding from the Australian government’s Medical Research Future Fund, Micro-X is advancing development of its portable Head CT scanner for use in a standard ambulance or retrieval aircraft to determine stroke type.
Weighing around 70kg – compared to conventional CT scanners that weigh more than 700kg – the Head CT is much smaller, lightweight and portable.
Micro-X recently announced it had been awarded a $4.4m grant by the Australian government’s Industry Growth Program to build and trial a world-first stroke-diagnosis-capable ambulance using its Head CT device.
The company has also been chosen to take part in a remote Australia air ambulance research study, funded by a $1.4m NHMRC Partnership Project grant led by ASA senior research fellow Dr Anna Balabanski.
The study is independent of, and will not impact, the planned timeline for Head CT regulatory submissions.
Head CT passes testing for human trials
The first Micro-X Head CT test bench for hospital trials has passed all engineering and safety testing.
It features a curved array of 21 miniature NEX tubes, advanced high-voltage switching electronics, and a world-first curved detector co-developed with Fujifilm.
Using Micro X’s novel sparse view approach, the system can create 3D images from fewer scans, which significantly lowers patient radiation exposure.
The dose is less than one millisievert – classified as very low and well below the recommended annual limit for the general population, which will be further validated in human trials.
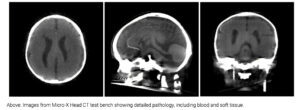
Image: Micro-X
On track for regulatory submissions in 2026
Micro-X CEO Kingsley Hall said completing its first Head CT imaging test system was the culmination of four years of commitment by its team in partnership with the ASA.
“This is a major milestone in the development of our world-leading Head CT scanner for stroke,” he said.
“We will now start installing our Head CT units in Australian hospitals to commence the ASA-led validation study that is designed to demonstrate our imaging in clinical applications and to image suspected stroke patients on their admission to hospital ahead of diagnosis.
“With our Head CT device in advanced development, we are on track for regulatory submissions to be made in 2026.”
‘Revolutionising point-of-care stroke diagnosis’
Chief operating officer Anthony Skeats said the company had now delivered the first CT solution that was low cost and offered image quality with the potential to transform any ambulance into a stroke-capable ambulance.
“This innovation opens the possibility of revolutionising rapid point-of care stroke diagnosis, saving lives and preventing permanent disability from timely intervention,” he said.
“In achieving this milestone, we have developed miniature NEX Technology tubes the size of golf balls, ten times smaller than our previous tubes.
“We have also invented fast switching high voltage electronics and novel image reconstruction software algorithms.”
Skeats said collaborating with ASA and partners Monash Health Collab, Johns Hopkins iStar labs and the South Australian Ambulance Service had helped bring the “game-changing product concept into reality”.
“We look forward to commencing our human imaging trials, to build the data to gain regulatory approval,” he added.
ASA co-chairs Professors Stephen Davis AO and Geoffrey Donnan AO said the organisation looked forward to the forthcoming first-in-world human stroke trials with the Micro-X test bench.
They said with a projected weight of 10% of the current, lightest CT scanner, successful human trials would enable prehospital deployment in regular ambulances and aircraft to move the hospital to the patient, treat stroke much earlier and reduce disability.
This article was developed in collaboration with Micro-X, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








