LTR Pharma successfully completes $25m cap raise to accelerate novel ED treatment

LTR Pharma is set to be well funded to support commercialisation of its ED treatment. Pic: Getty Images.
- LTR Pharma has completed a $25m placement to support Spontan through FDA regulatory pathway and commercialisation
- Placement supported by institutional and sophisticated investors and healthcare-focused funds
- LTR Pharma said it was now funded through until the end of 2026, which includes key upcoming value-creating milestones
Special Report: LTR Pharma has undertaken a $25 million placement to support the commercialisation of Spontan – its fast-acting nasal spray treatment for erectile dysfunction (ED) – through imminent regulatory milestones.
LTR Pharma (ASX:LTP) has announced it has received binding commitments for a $25 million placement, receiving strong support from institutional and sophisticated investors, as well as key healthcare-focused funds.
A total of 27,173,913 fully paid ordinary shares at 92cents/share will be issued, representing a 12.4% discount to the close of trading on December 5, 2024.
Settlement of the placement is expected to occur on December 13, 2024.
Bell Potter Securities Limited and Alpine Capital Pty Limited acted as joint lead managers for the placement.
Funds to support commercialisation of Spontan
LTR Pharma said funds raised from the placement would be used to accelerate the commercialisation and growth of its novel proprietary PDE5 nasal spray Spontan.
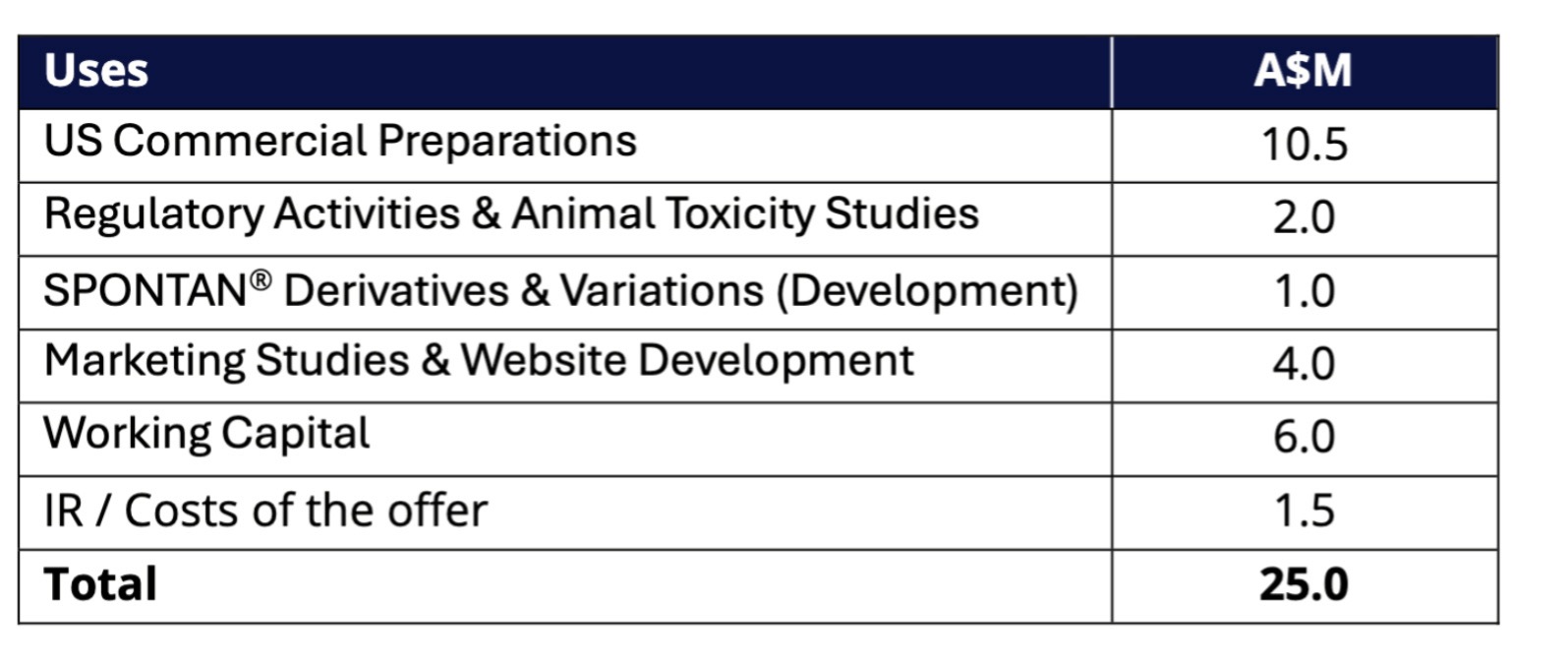 Source: LTR Pharma
Source: LTR Pharma
The company said it was now funded through until the end of 2026, which includes key upcoming value-creating milestones as Spontan advances towards commercialisation.
Positive trial results and key developments
In October LTR Pharma released results of its pivotal pharmacokinetic and trial of Spontan, which showed that the intranasal formulation to treat erectile dysfunction outperforms oral tablets.
Spontan showed 470% faster absorption than oral vardenafil of 12 minutes versus 56 minutes with the company believing its favourable safety profile positions the formulation as a disruptor in the global ED market.
Following these positive outcomes, Spontan has started early market access through Australia’s Therapeutic Goods Administration (TGA) Special Access Scheme and Authorised Prescriber Scheme, enabling it to be prescribed by healthcare professionals to patients with unmet needs.
The company has also inked a strategic global co-development deal with Aptar Pharma to support the commercialisation of Spontan in key markets, including the US.
LTR Pharma is now actively pursuing expedited regulatory pathways, including the FDA’s 505(b)(2) process in the US and Category 1 – Type F application with the TGA in Australia.
Strengthening its commercial position, the company established a joint venture with privately owned men’s healthcare group Restorative Sexual Health Clinic to develop an online healthcare platform, capitalising on the growing telehealth market and expanding access to men’s health services. A growing cohort of leading clinicians are now proactively embracing the opportunity to prescribe Spontan via early access pathways.
LTR Pharma said it was committed to addressing significant unmet needs in men’s health services and ED treatment, offering patients a fast-acting, on-demand solution that could transform the standard of care in this therapeutic area.
The company is also seeking to leverage its IP and invest in product R&D to expand its nasal spray product portfolio for ED and non-ED applications.
‘Beginning of the next transformative chapter’
LTR Pharma chairman Lee Rodne said the successful $25m placement was underscored by strong support from institutional investors.
“This funding is a critical milestone for LTR Pharma as we advance towards commercialising Spontan in the US, Australia and other key markets,” he said.
“With our preparations for regulatory engagement underway, and with our strategic partnerships, including our co-development agreement with Aptar Pharma, we are well-positioned to bring a first-in-class, rapid-onset treatment to market.
“This marks the beginning of the next transformative chapter for LTR Pharma as we look ahead to the commercialisation of Spontan and pioneering innovations in nasal spray therapeutics.”
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








