You might be interested in
News
Closing Bell: ASX slips from record highs, but Black Friday sales could be catalyst for next week
News
ASX Small Caps Lunch Wrap: CPI data put a rocket up the ASX, but REX is circling the drain
Health & Biotech
Health & Biotech
A drug that is implanted into the brain is effective against Parkinson’s, biotech Living Cell Technologies says.
The drug, called NTCELL, has come out of a one-year follow-up of patients who went through a Phase 2b trial.
Clinical trials are generally divided into three phases. Phase 1 focuses on safety, phase 2 tests effectiveness and Phase 3 examines whether it’s an improvement on existing treatments. Sometimes phases are divided into stages A and B, where B is generally more rigorous.
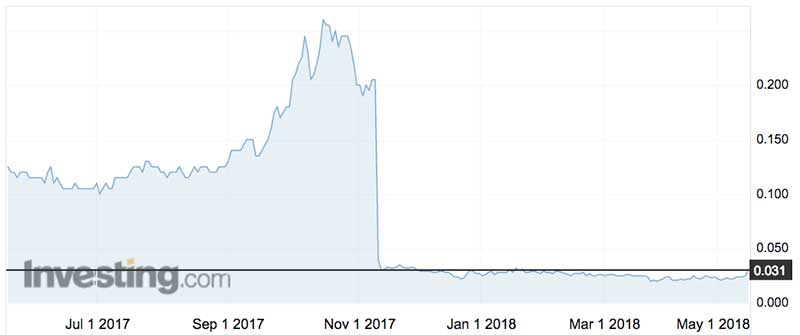
In November Living Cell’s share price collapsed by 80 per cent after it reported there was “not a statistically significant difference between the groups who received NTCELL and the patients who had sham surgery” 26 weeks after a Phase 2b trial.
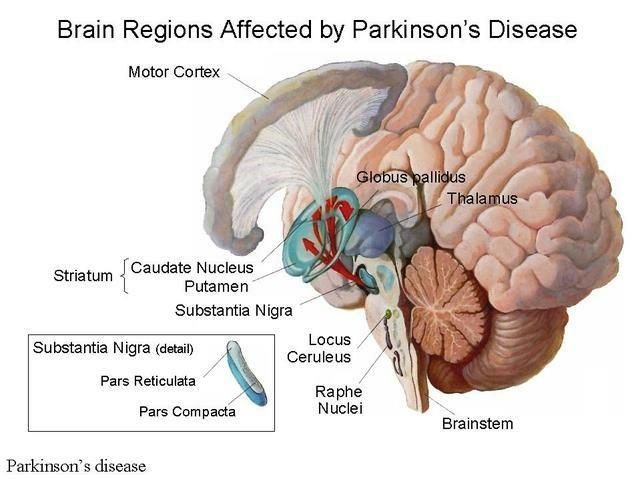
But new data from a one-year follow-up shows NTCELL created a “statistically significant improvement” in what’s known as the Unified Parkinson’s Disease Rating Scale, when patients had 40 to 80 NTCELL capsules implanted into the putamen — a round structure at the base of the forebrain — on both sides of the brain.
Living Cell’s shares jumped 52 per cent to 3.8c this morning — but the stock is still a long way from its one-year high of 28c.
Parkinson’s is a long-term degenerative disorder of the central nervous system that mainly affects the motor functions — that is movement. Symptoms emerge slowly over time and start with shaking and difficulty walking, and progress to dementia and mental health issues.
The Unified scale’s monitoring system is made up of six sections:
It’s estimated that 7 million to 10 million people have Parkinson’s worldwide, including 80,000 in Australia.
Three groups of six patients were in the latest study.
Two from each group had a sham surgery with no implants. Group one received 40 microcapsules, Group two 80 and Group three 120.
“The treatment is safe,” said principal investigator at Auckland City Hospital Dr Barry Snow.
“There are clinical signals of interest. We need to continue to monitor patients for longer to examine the clinical significance of this efficacy data.”
Living Cell chief Ken Taylor told Stockhead the trial has a two year follow-up period so they will be ready to start thinking about licensing or commercialization deals towards the end of this year, or by May 2019.
He said the trial had a six month deadline to find out if the drug worked and they passed that with unsatisfactory results.
But now they’re at the 12 month mark they are beginning to see the efficacy results they were expecting.
Living Cell Tech is running the trials exclusively in New Zealand, because the NTCELL drug is made from pathogen-free pigs from the remote sub-Antarctic Auckland Islands.
Mr Taylor says they are working with the New Zealand Ministry of Health but will look at registering their data with foreign health regulators once they have finished the trial follow-up period.
NTCELL works by coating choroid plexus cells from newborn pigs — the cells that produce cerebrospinal fluid in the brain — and turning that into a capsule that can be implanted into the brain.
The coating protects the cells from an anti-immune attack, and the cells support nerve cell functions and protective enzymes that are crucial for nerve growth and healthy functioning.
NTCELL functions as a neurochemical factory producing cerebrospinal fluid that promote new central nervous system growth and repair disease-induced nerve degeneration.