Imagion to start production of MagSense imaging agent for phase II breast cancer trial

The MagSense HER2 breast cancer imaging agent aims to detect cancer in lymph nodes. Pic via Getty Images
- Manufacturing to start on new batch of Imagion’s MagSense HER2 breast cancer imaging agent
- The new batch intended for use in phase II study to be proposed to US FDA in an Investigational New Drug application
- Imagion has previously made two batches of the imaging agent used in a phase I study on HER2+ breast cancer
Special Report: Imagion Biosystems has initiated manufacturing of its MagSense imaging agent for its phase II clinical study on HER2+ breast cancer, an essential requirement for filing an Investigational New Drug (IND) application with the US Food and Drug Administration.
Imagion Biosystems (ASX:IBX) noted that with a production schedule from the contract manufacture, the company can establish a timeline for completing the additional key tasks and forecasts being able to file the IND 30 to 60 days after manufacturing has been completed.
Based on the contract manufacturer’s availability of the GMP suite, manufacturing of the MagSense HER2 imaging agent is expected to start in mid-April, with production being completed in June.
Imagion said it was required to make the MagSense HER2 imaging agent for human clinical investigational use in compliance with FDA guidance for Good Manufacturing Practices (GMP).
The company has previously made two batches of the imaging agent, which were used in the phase I study on HER2+ breast cancer, where the key milestone of basic safety was achieved.
Imagion said the new batch was intended to be used in the phase II study that would be proposed to the FDA in the IND application.
For phase II, the company aims to optimise the dose of the imaging agent and the imaging protocol to establish the diagnostic performance.
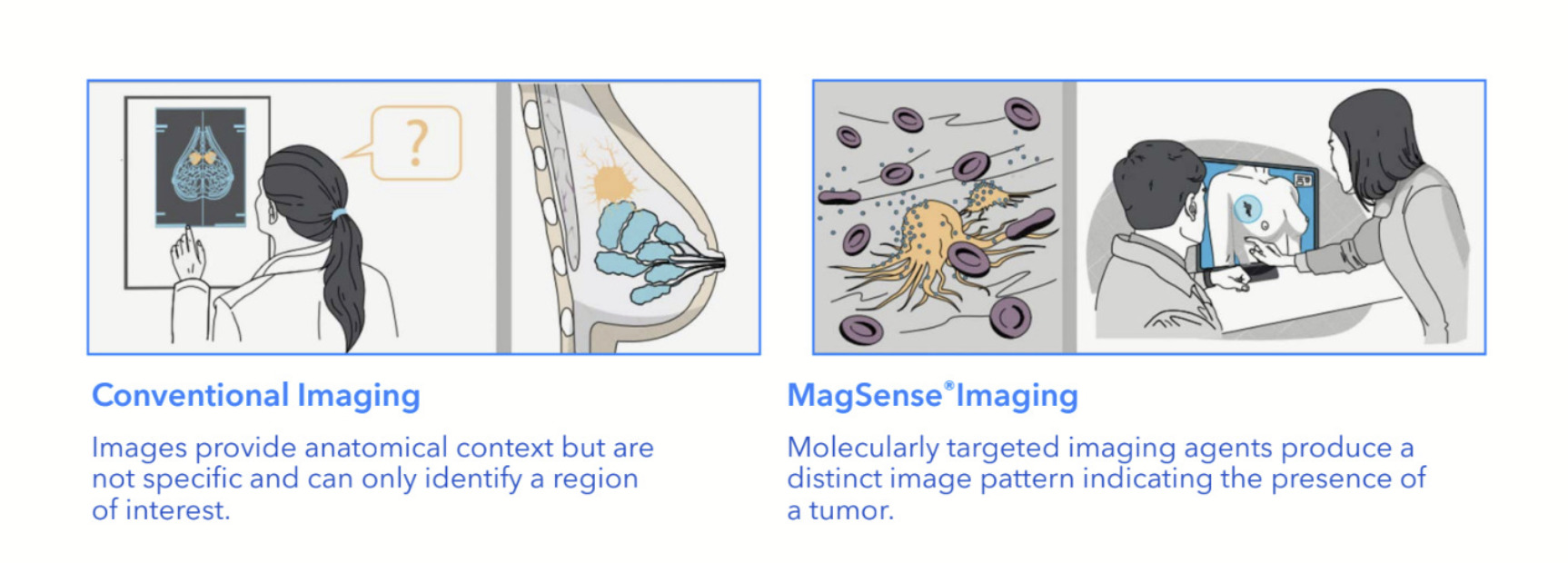
MagSense HER2 agent to detect cancer in lymph nodes
The MagSense HER2 imaging agent forms part of Imagion’s MagSense Imaging platform.
The platform aims to improve on current techniques for cancer diagnosis such as X-Rays, magnetic resonance imaging (MRI), computed tomography (CT), ultrasounds and positron emission tomography (PET) by providing a more specific and personalised approach.
It involves the use of iron oxide nanoparticles labelled with cancer-specific targeting antibodies, which can then be imaged with the widely available MRI.
Metastatic HER2+ breast cancer is known to be highly aggressive with a high rate of recurrence and metastasis with 400,000 new cases diagnosed globally each year.
The company said the phase II clinical study was a critical step in development of the MagSense HER2 imaging agent, intended to be used to detect cancer in the lymph nodes of patients with HER2+ breast cancer.
Precise nodal staging is an essential component in the management of patients with breast cancer, noted the company, adding that currently the most employed method for precise nodal staging is ultrasound, with pooled diagnostic sensitivity and specificity of 49% to 87% and 55% to 97%, respectively.
Given its aggressiveness, development of a more sensitive and specific imaging method for HER2+ breast cancer cases would be of significant clinical value, believes Imagion.
Well-funded to achieve filing of IND
Imagion noted that, along with manufacturing a new batch of the MagSense HER2 imaging agent, key tasks associated with meeting the IND milestone include:
- Establishing the principal investigator to lead the study
- Engaging with the principal investigator to finalise the study protocol in concert with FDA recommendations
Executive chairman Bob Proulx said the company has sufficient capital to proceed with the activities necessary to achieve filing of the IND, following Imagion’s recent $3 million capital raise.
However, he said additional funding would be needed to fund the phase II study once approved by the FDA.
“I’m very pleased that we have managed to get the manufacturing contract in place so quickly,” said Proulx.
“The receipt of funds from our recent capital raise has provided us with the resources needed to aggressively pursue our goal of filing an IND and preparing for the next phase of clinical testing of our novel imaging technology.
“With this key activity now in process, we are marching towards our next key development milestone.”
This article was developed in collaboration with Imagion Biosystems, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








