Imagion shares rally after positive FDA feedback on path to phase II breast cancer trial

Imagion edges closer to US phase II breast cancer trial after FDA feedback. Pic via Getty Images
- Imagion receives positive feedback from US FDA for HER2 breast cancer phase II clinical trial
- The company’s share price on the ASX has rallied early, more than 90%, with more than 100m shares traded
- Imagion’s clinical team set to meet with regulator in coming week to complete formal review process
- Company plans to file the investigational new drug application for phase II trial in Q3 CY2
Special Report: Imagion Biosystems is moving closer to initiating its phase II clinical trial for HER2-positive breast cancer in the US after receiving formal written feedback from the US Food and Drug Administration (FDA).
Imagion Biosystems (ASX:IBX) said the FDA had provided constructive input on its MagSense HER2 imaging agent study helping to shape the clinical trial design and expected outcomes.
The feedback is part of the standard review process for FDA approved clinical trials.
IBX’s clinical team is now set to meet with the FDA in person in the coming week to finalise the formal review process for the phase II trial ahead of an investigational new drug (IND) application.
Imagion plans to file the IND application for the phase II study in Q3 CY25.
“I’m very pleased with the trajectory of our communications with the FDA,” said IBX executive chairman Bob Proulx.
“We view the feedback from the FDA as very encouraging and can now confidently press forward with the formal submission and plans for undertaking the phase II clinical study, knowing we are in good shape regarding the regulatory path.”
Listen: Bob Proulx joins Tim Boreham on the Health Kick podcast
Phase II trial critical step in MagSense development
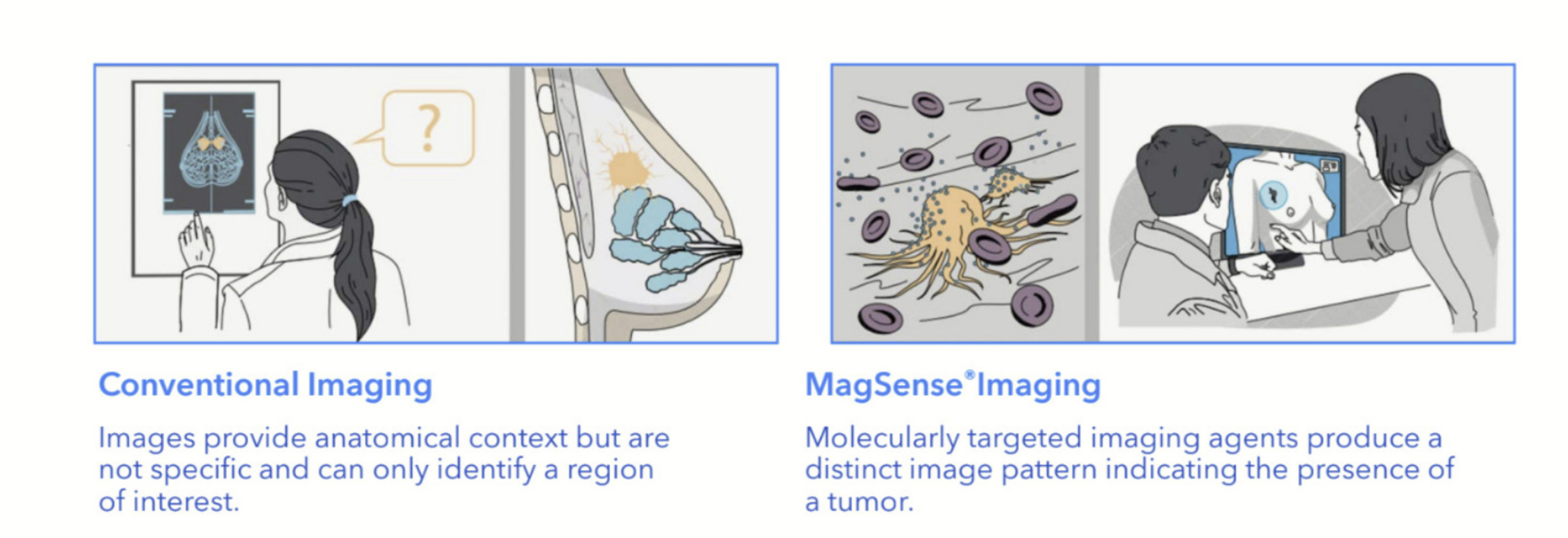
The MagSense HER2 imaging agent forms part of Imagion’s MagSense Imaging platform.
The platform aims to improve on current techniques used for cancer diagnosis such as X-Rays, magnetic resonance imaging (MRI), computed tomography (CT), ultrasounds and positron emission tomography (PET) by providing a more specific and personalised approach.
The platform involves the use of iron oxide nanoparticles labelled with cancer-specific targeting antibodies, which can then be imaged with the widely available MRI.
Metastatic HER2+ breast cancer is known to be highly aggressive with a high rate of recurrence and metastasis with 400,000 new cases diagnosed globally each year.
Imagion said the phase II clinical study was a critical step in development of the MagSense HER2 imaging agent, intended to be used to detect cancer in the lymph nodes of patients with HER2+ breast cancer.
Precise nodal staging is an essential component in the management of patients with breast cancer with currently the most employed method for precise nodal staging ultrasound, with pooled diagnostic sensitivity and specificity of 49% to 87% and 55% to 97%, respectively.
Imagion believes given its aggressiveness, development of a more sensitive and specific imaging method for HER2+ breast cancer cases would be of significant clinical value.
This article was developed in collaboration with Imagion Biosystems, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








