Green whistle maker Medical Dev drops 17pc after revenue warning

Johnathan Thurston holds onto a green whistle after an injury in the 2011 NRL State of Origin. Pic: Getty
The maker of Australia’s famous “green-whistle” pain reliever has made ten separate announcements on European approvals this year – but investors have been told revenue is still a work-in-progress.
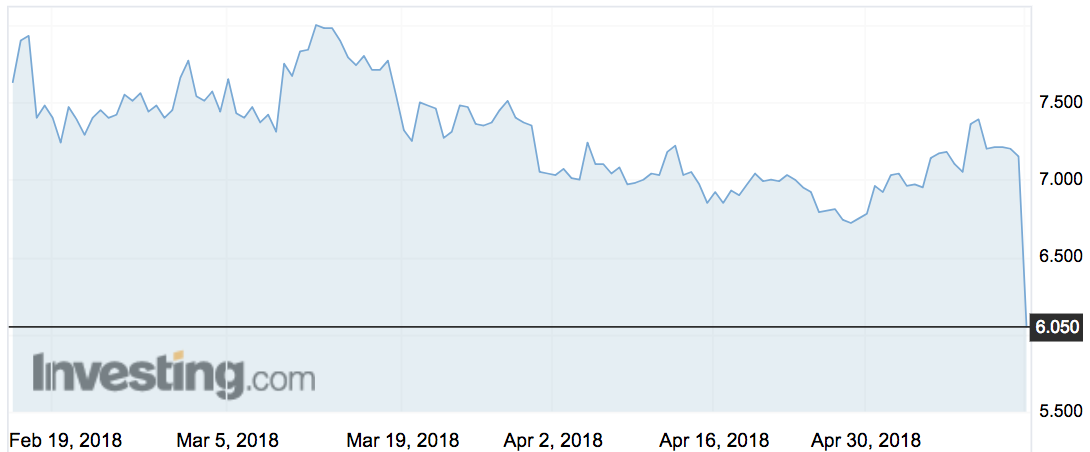
Medical Developments (ASX:MVP) shares dropped as much as 17 per cent today after the drug developer admitted sales were lower than expected this year.
Medical Dev is pursuing an aggressive global rollout of its Penthrox green whistle device — a self-administered, non-opioid pain relief inhaler seen used above by NRL player Johnathan Thurston.
Medical Dev chief John Sharman told investors that despite the global expansion plans – revenue for 2018 would likely be flat or slightly down from last year.
“FY18 Sales revenue is lower than market expectation mainly because of lower device sales in Australia and lower-than-expected sales growth rates for devices in the USA and Europe,” Mr Sharman said.
“Both the USA and Europe respiratory device businesses will still deliver good sales growth, but some delays in key contracts and partnership agreements have impacted our initial growth rate expectations.
“Underlying profit for FY18 is expected to be $0.3m because of, among other things, the company continues to invest in the required infrastructure ahead of the anticipated roll out of Penthrox across Europe, Mexico, Canada and the Middle East.”
The shares hit $5.92 this morning before rebounding to $6.22 by 12.20pm AEST.
The stock has traded between $4.61 and $8.07 over the past year. Medical Dev is valued at about $425 million.
- Bookmark this link for small cap breaking news
- Discuss small cap news in our Facebook group
- Follow us on Facebook or Twitter
- Subscribe to our daily newsletter
Sales in new markets were not expected until late 2019.
Earlier this year the company inked a US deal with the country’s second biggest drugstore chain, Walgreens and in September it was recommended for use in all UK ambulances for injuries causing moderate to severe pain.
Penthrox has been used safely and effectively for more than 40 years in Australia with some 5 million units sold.
MVP says there is growing interest in Penthrox being used in patients undergoing investigatory procedures such as colonoscopies.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








