First patient dosed with Imugene’s lung cancer drug candidate
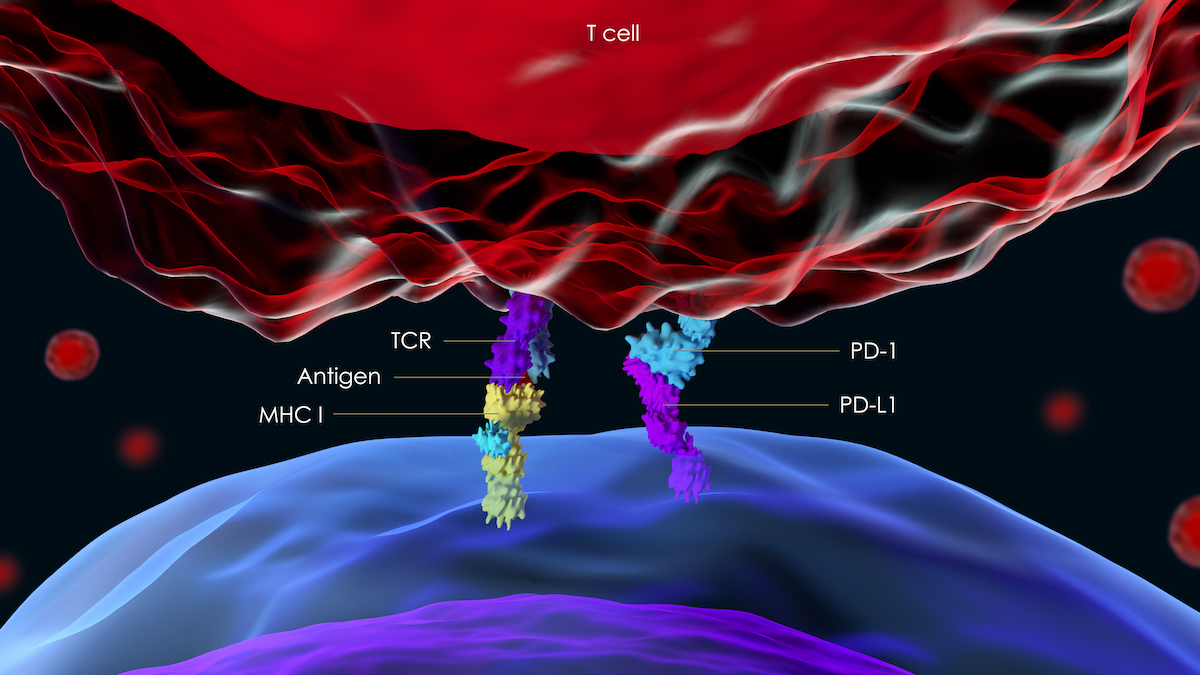
A rendering showing interactions between a T-cell (top) and a cancer cell (bottom). Getty Images.
Sydney biotech company Imugene (ASX:IMU) says it has dosed its first patient with its drug candidate PD1-Vaxx, a checkpoint immunotherapy designed to help cancer tumours meet “programmed death”.
The patient was dosed in Melbourne as part of Imugene’s phase 1 dose-escalation study, which is recruiting patients with non-small cell lung cancer.
PD1-Vaxx has shown great promise in mouse studies and has a similar potential mechanism of action as Merck & Co‘s (NYSE:MRK) “miracle drug” Keytruda and Bristol Myers Squibb’s (NYSE: BMY) Opdivo.
Some cancer cells produce a ligand to hide them from what’s known as the “programmed cell death-1” receptor in the body’s T-cells, thus evading the body’s normal anti-cancer response system. PD1-Vaxx aims to induce the body to produce polyclonal antibodies that block this signaling, so the cancer cells have nowhere to hide.
“The concept of teaching and inducing the body to generate its own antibodies targeting PD-1 expressing cells represents a paradigm shift in oncology, and is a novel treatment method for cancer,” says Imugene managing director and chief executive Leslie Chung said.
Up to 32 participants in Australia and the United States will be dosed with the PD1-Vaxx as part of the study, which aims to find the highest safe dose level that triggers the best immune response.
An estimated 12,800 Australians are diagnosed with lung cancer each year, according to the Australian Lung Foundation.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








