FDA reviews Aussie drug that helps people who are allergic to sunlight

The FDA is looking at an Australian drug that helps people who can never be exposed to light.
People who carry the very rare genetic blood disorder erythropoietic protoporphyria (EPP) can’t go into any kind of light, be it sunlight or that from a lamp.
The condition causes extreme biochemical reactions when skin is exposed to any light source, although sunlight is the worst.
After exposure, skin will start itching or become red, and at worst can actually burn.
Sufferers are forced to live entirely in the dark.
- Bookmark this link for small cap breaking news
- Discuss small cap news in our Facebook group
- Follow us on Facebook or Twitter
- Subscribe to our daily newsletter
Australian company Clinuvel (ASX:CUV) has been developing a drug for the last 14 years called SCENESSE.
It’s taken every two months and activates melanin, the pigment in the skin that causes tanning, to provide a physical barrier to shield the chemicals in the blood from the light.
The drug is already able to be used in Europe for adults, and Clinuvell hopes the FDA will finish its review in 2019 so it can start distributing there.
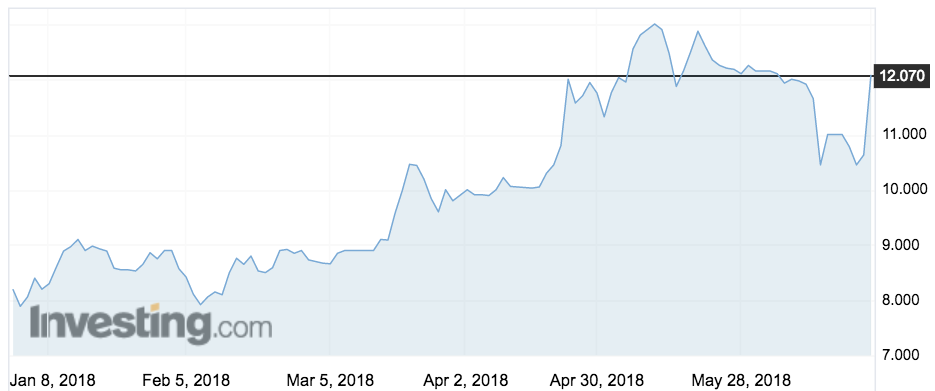
Clinuvel shares rose 14 per cent on the news to $12.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








