EMVision readies UCLA Health as final pivotal trial site for emu stroke detection device

The final site for EMVision’s pivotal trial is set to be activated in September. Pic via Getty
- Leading US stroke centre UCLA Health scheduled for site activation in EMVision’s pivotal trial of emu brain scanner for stroke
- UCLA Health final site to be activated in trial, joining three US and two Australian comprehensive stroke centres already recruiting
- Pivotal trial steering committee (TSC) assembled, comprising international key opinion leaders in stroke
Special Report: Final site activation in the pivotal validation trial for EMVision Medical Devices’ first commercial device – the emu point-of-care bedside brain scanner for stroke diagnosis – is scheduled for early September.
Leading US academic health and stroke centre UCLA Health is set to join the EMVision Medical Devices (ASX:EMV) pivotal trial with site activation scheduled in two weeks and the device now shipped.
First patient enrolments are expected shortly after activation with UCLA Health a major referral hub for complex neurological and cerebrovascular care.
The trial at UCLA Health will be led by principal investigator Dr May Nour, a trained vascular and interventional neurologist and medical director of California’s first mobile stroke unit (MSU).
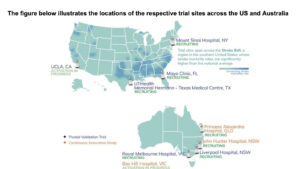
UCLA joins four US and two Australian comprehensive stroke centres that are actively progressing towards the recruitment target for H1 CY26.
Source: EMVision
About the pivotal trial
The pivotal trial is a prospective, multicentre, blinded study being undertaken in the US and Australia evaluating the diagnostic performance of the emu brain scanner in patients with suspected acute stroke.
The primary endpoint assesses accuracy against standard neuroimaging (CT/MRI), including sensitivity and specificity for haemorrhage detection.
Data from the trial will support EMVision’s planned de novo (new device) submission to the US Food and Drug Administration (FDA).
After initial training verification, the study runs in two arms including
- Arm A – 150 intracranial haemorrhage (ICH) patients; and
- Arm B – 150 suspected stroke patients.
Initially, all suspected stroke patients are enrolled in the appropriate arm. Enrolment then focuses on confirmed ICH patients, facilitated by the emu scanner’s integration with radiology bays, enabling rapid identification and scanning immediately after baseline CT.
All sites handle high patient volumes, with each treating at least 2,000 stroke patients and 300 ICH patients annually
Trial steering committee established
A trial steering committee comprising international key opinion leaders in stroke has been assembled to provide independent oversight and strategic guidance with members including:
- Co-chairs Professors Geoffrey Donnan AO and Stephen Davis AO. Donnan and Davis are also co-chairs of the Melbourne Mobile Stroke Unit program and the Australian Stroke Alliance
- Chief of stroke and cerebrovascular disease, Harvard Medical School and Beth Israel Deaconess Medical Center Professor Magdy Selim
- Director of the Intracerebral Haemorrhage Program at Mount Sinai Health System in New York Associate Professor Christopher Kellner
- Professor of Clinical Neurosciences, University of Calgary, and Provincial Medical Director for Neurosciences in Alberta Michael Hill
“We are fortunate to collaborate with institutions setting the global standard for stroke care and a Trial Steering Committee anchored in world-class expertise,” EMVision CEO and co-founder Scott Kirkland said.
Watch: CEO Scott Kirkland
Box Hill Hospital added to Continuous Innovation Study
Recruiting alongside the Pivotal Trial, EMVision’s Continuous Innovation Study continues at Princess Alexandra Hospital in Brisbane and John Hunter Hospital in Newcastle.
Box Hill Hospital has now been approved as a third site and is in the process of activation.
Recognised internationally with World Stroke Organisation Gold status, EMVision said Box Hill Hospital supported the study’s goal of advancing additional features and future indications for the emu device, including point-of-care traumatic brain injury assessment.
The Continuous Innovation study is key to the ongoing development and commercialisation of EMVision’s technology.
This article was developed in collaboration with EMVision, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.