Distribution deal opens new international opportunities for MGC Pharma

Pic: Charnchai / iStock / Getty Images Plus via Getty Images
Special report: A new distribution deal is opening up doors across Europe, the Middle East and North Africa for Australian medical cannabis developer MGC Pharma.
The company (ASX:MXC) told investors today it has inked a three-year agreement with one of the leading healthcare distributors across Europe and the Middle East – A.M. Mangion.
Under the terms of the deal, MGC will supply an initial range of its pharmaceutical-grade medical cannabis products for distribution via A.M. Mangion’s existing international network.
This includes Malta, Italy, France, Spain, Portugal, the UK, as well as the Middle East and North Africa.
Additional products added in the future.
Realising its ‘seed to pharma’ strategy
MGC Pharma was the first company to receive a binding letter of intent from Malta Enterprise to set up a medical cannabis cultivation and production facility in the country. Aurora
Architectural plans have already been completed and the master plan is in the works, with plans to begin construction of the facility in the next four to six weeks.
The company will use its expertise from establishing its GMP-certified facility in Slovenia over the last 18 months to fast-track its progress in Malta.
The facility’s GMP certification ensures that it meets strict EU requirements and makes it one of the most sophisticated facilities in the global medicinal cannabis industry.
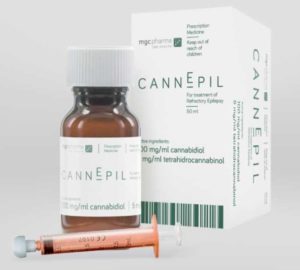
It allows for the immediate production of CannEpil, MGC’s treatment for refractory epilepsy as well as new product development and demonstrates its ‘seed-to-pharma’ capabilities.
Refractory epilepsy is when medicine is unable to bring a patient’s seizures under control.
Once built, MGC plans to cultivate all THC and CBD strains of medical cannabis at its new facility in Malta, allowing for the seed-to-pharma strategy to supercharge the company’s growth in Europe and internationally.
“I’m pleased with the significant progress we’ve made in establishing our facility on the land granted to us,” said MGC co-founder and Managing Director Roby Zomer.
“I am confident the steep learning curve the management team has undergone in establishing and now operating our very own GMP-certified facility in Slovenia gives us a strong competitive edge.”
This special report is brought to you by MGC Pharma.
This advice has been prepared without taking into account your objectives, financial situation or needs. You should, therefore, consider the appropriateness of the advice, in light of your own objectives, financial situation or needs, before acting on the advice.
If this advice relates to the acquisition, or possible acquisition, of a particular financial product, the recipient should obtain a Product Disclosure Statement (PDS) relating to the product and consider the PDS before making any decision about whether to acquire the product.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








