Dengue surges as climate change bites but one Aussie drug trial has slapped symptoms

Dengue fever has exploded as climate and urban sprawl fuel global spread. Picture via Getty Images
- Dengue cases explode as climate change and urban sprawl fuel surge
- World scrambles as virus spreads with no easy fix
- Island Pharma’s dengue drug cuts symptoms in Phase 2 trial
There’s a buzz in the air. You might not notice it at first, but it’s becoming harder to ignore.
It belongs to mosquitoes, and they’re not just a nuisance anymore. They’re carrying a virus that’s spreading fast, breaking out of the tropics and catching the world off guard.
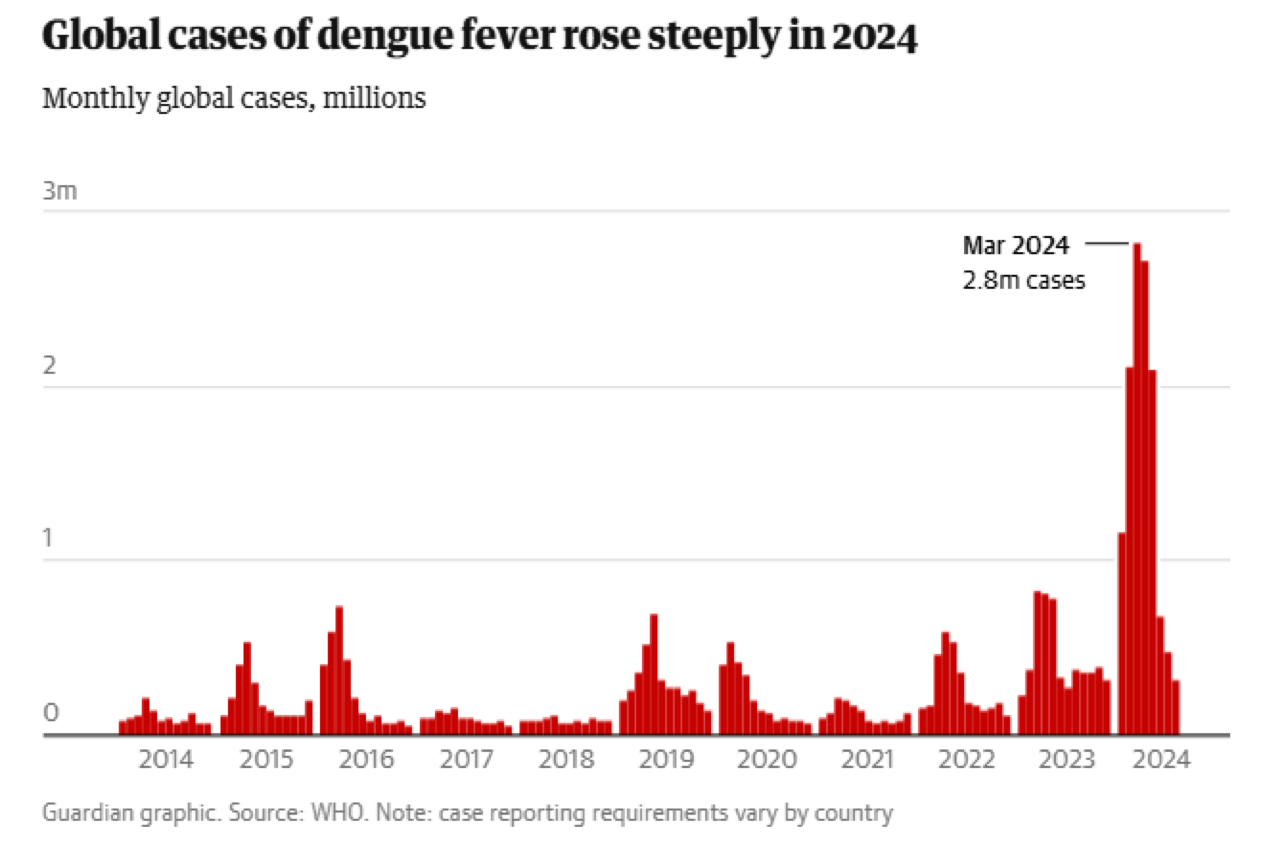
Dengue fever, once considered a regional menace, has gone global.
In 2023, the world recorded over 6 million cases. In 2024, they have exploded to as high as 2.8 million cases a month.
So what’s going on?
According to Professor Zulkifli Ismail, Chairman of the Asia Dengue Voice and Action Group (ADVA), a perfect storm is brewing.
“There are a number of things that are thought to drive this increase. The most popular of which is climate change and global warming that favours the mosquito breeding habits,” he told Stockhead.
Rapid urbanisation and more new construction sites also create new water collections for Aedes eggs. In addition, inadequate vector control causes an increase in the Aedes mosquito, the Professor added.
“An example is fogging that’s meant to kill adult mosquitoes, but inevitably also kills frogs that feed on the mosquitoes and larvae.”
People’s habits also play a role. When we don’t take care of the environment or delay seeing a doctor when symptoms start, it gives the virus more chances to spread.
“It’s thought that almost 70% of dengue cases are asymptomatic, meaning that these are potential mosquito-bite victims that can help to transmit the virus.”
Connection between climate change and dengue
Dengue travels through two species of mosquitoes: Aedes aegypti and Aedes albopictus, who act like little flying syringes.
These insects are cold-blooded, which means their biology speeds up with heat.
Climate change is therefore making conditions more ideal for them.
Warmer temperatures are helping them breed faster, live longer, and spread into regions that were once too cold for outbreaks to take hold.
A recent study spanning 21 countries found that climate change alone is responsible for about 20% of dengue’s rise from 1995 to 2014.
And that’s in regions where dengue was already endemic, like Brazil, Indonesia and India. The outlook is worse for parts of the world that are just now entering mosquito-friendly territory.
“There are certain ‘hot spot’ areas and these are related to the density of the Aedes aegypti and, to an extent, the Aedes albopictus mosquitoes that can transfer the virus from one person to another,” said Professor Ismail.
And then there’s the world’s urban sprawl where rubbish builds up. That, plus global travel, is a recipe for outbreaks.
Not just “breakbone fever”
Dengue earned the nickname “breakbone fever” because of the intense muscle and joint pain it can cause, which makes you feel like your bones are cracking.
In severe cases, dengue can lead to internal bleeding, low platelet counts, and even organ failure. It can also affect the nervous system, causing conditions like encephalitis or Guillain-Barré syndrome.
There are four types of the virus, so getting dengue once doesn’t mean you’re safe. Repeat infections can be worse. And there’s still no widely available treatment.
Supportive care and fluids are the standard. Vaccines exist, but they’re complex and not easy to access.
Professor Ismail’s group, ADVA, helps countries improve their local responses by sharing expertise and encouraging more coordinated action.
Some countries, like Indonesia and Malaysia, have tried using Wolbachia-infected mosquitoes to reduce the spread of dengue.
The results have been good, but the method is expensive and not easy to roll out everywhere.
Hope from Australia’s Island Pharma?
With the virus on the march and few medical options on the table, an Aussie biotech called Island Pharmaceuticals (ASX:ILA) is starting to draw attention.
Its lead candidate, ISLA-101, is a repurposed antiviral.
It was originally developed for other uses, including cancer, but is now being trialled as a potential treatment and preventative for dengue fever.
The drug works by stopping a key viral protein from entering the nucleus of human cells.
That step is essential for the virus to replicate. If the protein can’t get in, the virus can’t multiply. It’s a clean, targeted mechanism, and so far, it’s showing signs of promise.
Island recently completed its Phase 2a/b PROTECT trial in the US, using a dengue human infection model.
This model is as close as possible to a real-world scenario, but within a controlled environment.
Healthy volunteers were given either ISLA-101 or a placebo, then deliberately exposed to a weakened strain of dengue virus. This strain, provided by the US Army, causes mild to moderate symptoms and allows researchers to study viraemia and drug response safely.
More on Island’s trials
The trial was split into two parts.
In the preventative arm (Phase 2a), participants received ISLA-101 three days before virus exposure.
The results were encouraging. Those given the drug had a clear drop in viral load and reported fewer symptoms than those on placebo.
On average, the placebo group reported around 63% of all possible dengue symptoms, while those given ISLA-101 reported about 33%.
This suggests the drug may help prevent or reduce the severity of infection when taken early.
In the treatment arm (Phase 2b), participants were exposed to dengue first, then given ISLA-101 seven days later.
There were signs the drug still had an effect on viral replication, but because some participants were already showing symptoms at the time of dosing, changes in clinical outcomes were less consistent and are still under analysis.
Importantly, the trial met its key objectives. It confirmed safety, reached the target drug concentration in blood, and showed early signs of antiviral activity.
The results were strong enough to warrant deeper clinical investigation.
Read later: Island backs ISLA-101 dengue potential with top-line Phase 2a/b data
The world is watching, and waiting …
Professor Stephen Thomas, who helped oversee the trial, said the dengue problem is worsening, and the number of candidate countermeasures in clinical testing does not align with the global scope of the problem.
“For this reason, we are very excited Island’s candidate compound demonstrated evidence of antiviral activity against a rigorous dengue human infection model. These results set the stage for continued clinical development.”
The study also involved a strong group of collaborators, including Island, Upstate Medical University, the Walter Reed Army Institute of Research, and the US Army’s Medical Research Program.
With these Phase 2a/b results now in hand, Island is reviewing the data with its scientific and clinical advisors to plan the next stage.
This could include a larger study and more detailed analysis of both prevention and treatment use cases.
If ISLA-101 continues to show clinical benefit, it may become eligible for a Priority Review Voucher from the FDA.
These vouchers allow faster approval for future drugs or can be sold to other companies, often for over US$110 million.
“If [Island Pharma’s] antiviral can convincingly reduce the viraemia and also mitigate severe dengue, it will be one of the tools in our armamentarium to treat dengue cases,” said Professor Ismail.
It’s still early, but in a world with millions of dengue infections each year and no widely available treatment, Island’s progress is worth paying close attention to.
At Stockhead we tell it like it is. While Island Pharmaceuticals is a Stockhead advertiser, it did not sponsor this article.
This story does not constitute financial product advice. You should consider obtaining independent advice before making any financial decision.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








