Cancer trial to test Noxopharm’s Veyonda with Opdivo
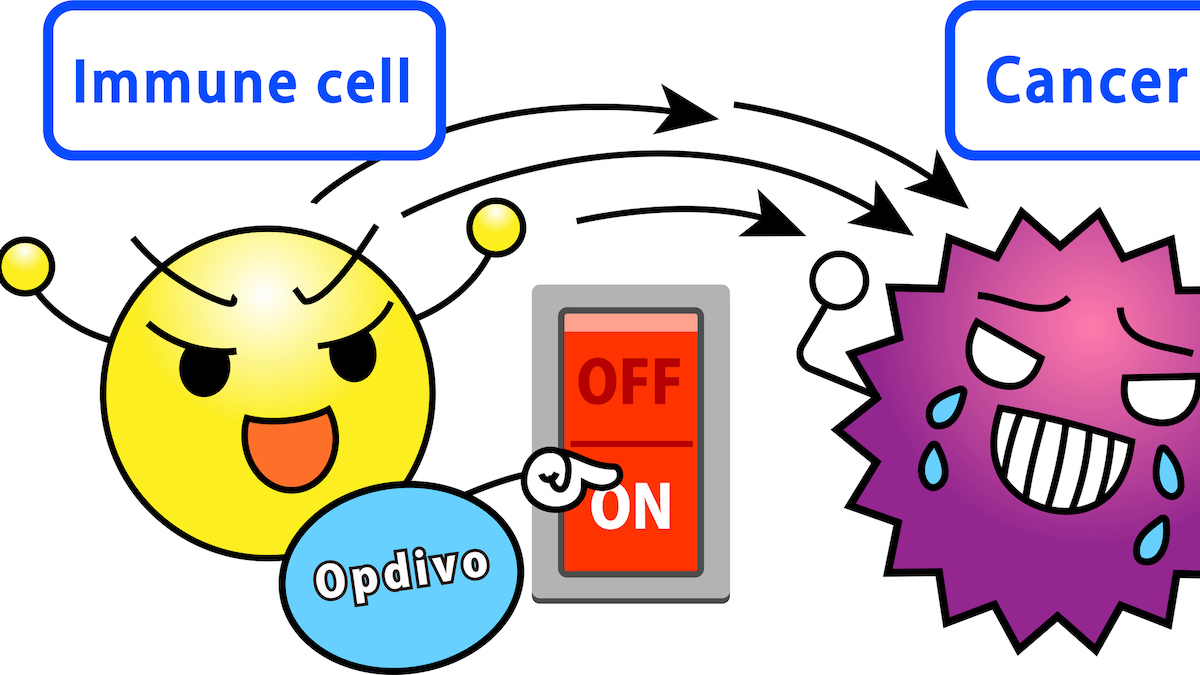
Opdivo fllips a switch in the body's immune system so cancer can't hide ... and Noxopharm hopes its suppository can make the immunotherapy even more effective. (Getty Images)
A phase 1b clinical trial testing the safety and efficacy of Noxopharm (ASX:NOX)‘s Veyonda suppository when combined with Bristol Myer Squibb’s immunotherapy drug Opdivo (nivolumab) has received ethics approval.
The IONIC-1 trial will immediately begin recruitment in Australia, with a goal of enrolling 30 cancer patients.
“If we’re able to enhance the effects of BMS’s nivolumab with Veyonda, this will bring some truly life-changing treatment to cancer patients’ lives and will change the way that cancer is treated worldwide,” said Dr Paul De Souza, principal investigator and dean of medicine at the University of Wollongong.
Along with Merck’s Keytruda, Opdivo is one of two blockbuster cancer “checkpoint inhibitors” drugs, which work by preventing cancer cells from hiding from the body’s immune system.
But while these drugs have dramatic benefits in some cancers, others have proven resistant, and scientists believe that’s because some individual tumours actively expel immune cells.
Noxopharm is hoping that Veyonda, which can help restore immune function in tumours, will allow Opdivo to be used against even more kinds of cancers.
Bristol Myers Squibb reported $US6.99 billion ($9 billion) in Opdivo sales last year, making it the company’s third-most valuable drug.
Noxopharm shares were unchanged at 68.5c at 11.02am on Friday.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








