Cancer fighter Invion offloads respiratory drugs into China spin-off

Invion — one of the ASX’s best-performing biotech stocks — is ridding itself of its legacy respiratory drugs and packing them off to China.
The cancer fighter (ASX:IVX) will spin off its INV102 (nadolol) and INV104 (zafirlukast) drug candidates into an unlisted company called Chronic Airway Therapeutics.
The drugs will be developed in China with plans to undergo Phase 3 trials in 2019.
(Clinical trials are generally divided into three phases. Phase 1 focuses on safety, Phase 2 tests for effectiveness and Phase 3 examines whether the new drug is an improvement on existing treatment.)
Invion says it’s been trying to sell or license out the technologies since 2015.
“We’ve been saying all along … that people are struggling to see the path forward with a drug that’s already generic, that has intellectual property around a particular dosing schedule and is contrary indicated in the disease,” Invion boss Greg Collier told Stockhead.
Invion had “trawled around the US and Europe to try to find partners” but no one was interested, and they recently saw an opportunity in China where regulatory changes have made it easier to get drugs approved, Dr Collier said.
The drug will be aimed at chronic obstructive pulmonary disease (COPD), an illness that Dr Collier says affects over 100 million Chinese adults.
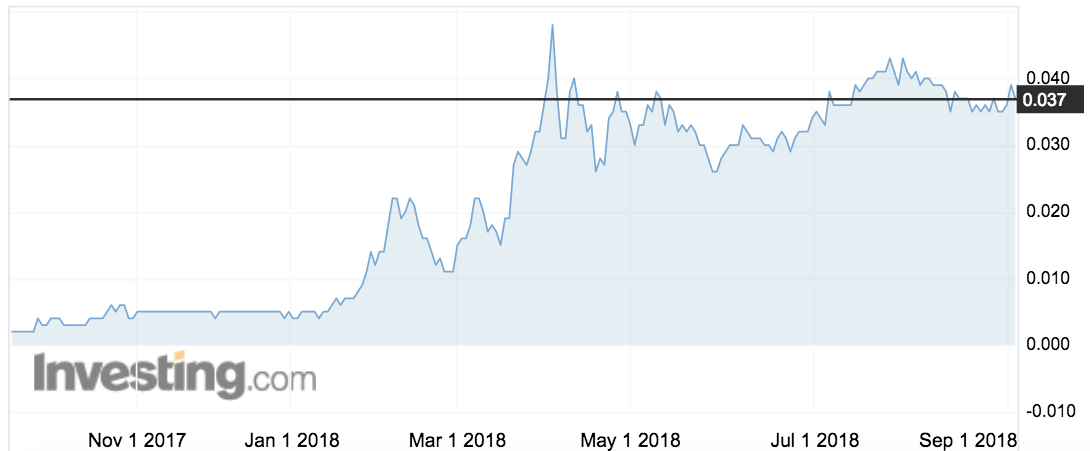
Invion is one of the best performing biotech small caps over the past year. The stock has skyrocketed from 0.2c to around 3.7c over the past year:
Invion acquired Nadolol via a merger in 2012 which promised “smoking cessation” and to help with asthma.
While it helped reduce the number of cigarettes consumed, more work was apparently needed on the “cessation” part.
It is the one the new company will be focusing on. There are no plans to advance zafirlukast.
Both have been overshadowed by an exclusive distribution and licence agreement last year to commercialise and develop a light-based cancer treatment called Photosoft from Chinese Cho Group.
Cancer just the start
The therapy is starting with ovarian cancer but has applications across prostate, skin, lung and other cancers as well, says Dr Collier.
After setting up an Australian manufacturing base to make oral and intravenous version of a drug that is activated by a laser, they plan to start clinical trials in the first quarter of next year.
“That drug is activated by certain wave lengths of light, that form oxygen free radicals that kill the cells and set up an immune response in the body,” he explained.
Cho is the corporate vehicle of the Melbourne-based entrepreneur Michael Cho.
- Subscribe to our daily newsletter
- Bookmark this link forsmall cap news
- Join our small cap Facebook group
- Follow us on Facebook or Twitter
In a company-making move, a year ago Invion issued Cho Group $5.5 million in shares at 0.2 cents and Cho agreed to underwrite a $2.5 million rights issue at the same price.
Cho now owns 68 per cent of Invion.
Invion shareholders will receive one share in the new company for every one held in the parent company, but there is in intention to list it.
Cho Group is lending the new company $200,000 to fund its launch and Investigational New Drug (IND) applications with the Chinese Food and Drug Administration (CFDA).
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








