Botanix is developing a game plan for a new drug that could fight skin superbugs

Pic: Godji10 / iStock / Getty Images Plus via Getty Images
Dermatologist Botanix Pharmaceuticals says pre-clinical testing of two of its key products show significant potential for solving serious skin infections and eliminating strains of antibiotic-resistant bacteria.
Botanix (ASX:BOT) updated investors on Monday on the clinical trial process for two of its drug projects, BTX 1801 and BTX 1308.
BTX 1801 is Botanix’s newest product, an anti-microbial which the company believes could “address the significant global public health issue of antimicrobial resistance”.
Botanix believes the drug has significant potential to fight serious skin conditions, with early tests indicating the product could kill off high levels of bacteria.
The drug could eventually be used to target treatments to a range of skin conditions, including acute bacterial and skin structure infections, which hospitalise three million people a year.
The company will now work with “key opinion leaders” to develop a market review for the use of BTYX 1801, including deciding on a key skin infection to test the product on initially, and developing a research pathway.
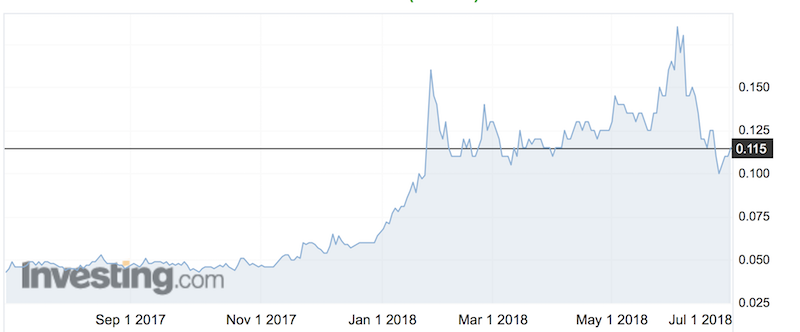
Shares in Botanix are up 167 per cent over the past 12 months. Shares were up half a cent to 11.5c by 11am AEST on news of the product plan.
The company also confirmed on Monday it was advancing its patient studies for psoriasis treatment BTX 1308 after pre-clinical tests gave evidence the drug addressed inflammation and bacterial infection.
A study will commence in Europe in the third quarter of 2018.
- Subscribe to our daily newsletter
- Bookmark this link for small cap news
- Join our small cap Facebook group
- Follow us on Facebook or Twitter
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








