Bioxyne’s revenue soars 48pc after key manufacturing licences boost growth
Health & Biotech
Health & Biotech
Special Report: Medicinal cannabis products supplier Bioxyne is on the up today after reporting a strong start to the new financial year with rapid growth following granting of key licences.
Releasing its Q1 FY25 report, Bioxyne (ASX:BXN) said within a short period the company had made outstanding progress toward becoming Australia’s leading manufacturer and wholesaler of novel medicines, complementing sale and distribution of its Dr Watson health products.
BXN was granted a Good Manufacturing Practice (GMP) licence to manufacture cannabis by the Therapeutic Goods Administration (TGA) in February 2024.
The company was also granted Australia’s first GMP licence for psilocybin and MDMA (3,4-methylenedioxy-methamphetamine).
Other highlights of the quarter include:
BXN said group revenue from the sale of its trademarked Dr Watson health supplements, and the wholesale of the company’s probiotic Lactobacillus Fermentum PCC, were in line with expectations.
The company said outperformance came from the newly established manufacturing capacity and improved operations.
BXN said its focus was on profitable growth. By maintaining an increase in revenue and managing operating costs, the company is aiming to achieve its aim of profitable and cash flow positive trading in each quarter of FY25.
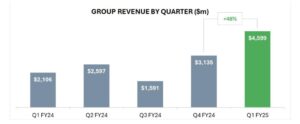
BXN group revenue by quarter ($m). Source: BXN
BXN started its first commercial manufacture of pharmaceutical-grade edible cannabis pastilles, often referred to as gummies or edibles, in July 2024.
The company said this included supply of edible pastilles to one of Australia’s top five alternative authorised prescriber medicine clinics, by its wholly owned subsidiary
Breathe Life Sciences (BLS).
BXN has forecasted a minimum supply to the clinic of $28m worth of edible cannabis products over the next 24 months.
The company secured late in Q1 a further major manufacturing agreement with Aura Therapeutics, with potential annualised revenue of $5.94m, to supply both THC flower products and edible cannabis pastilles.
BXN said further supply contracts were currently in negotiation with the company now having capacity to manufacture 12,000kg of THC flower products and 24 million THC pastille products annually for distribution to Australian and overseas customers.
The company’s planned expansion of its manufacturing capabilities for pastilles and flower products is scheduled to come online by December 2024, tripling its current output.
BLS said it would also continue to manufacture and supply Australian, New Zealand, US, and European clinical trials with novel psilocybin products to develop proprietary formulations and drug delivery methods, as well as supply Australian patients via the TGA’s Authorised Prescriber Scheme.
BXN said it was focused on growing its manufacturing customer base, expanding the market for its Dr Watson health and wellness products for consumers, and introducing its proprietary probiotic product into its existing global distribution channels.
The company said its current order book for supplying customer companies continues to grow as it scales operations while maintaining the high-quality standards that BLS has provided with its pharmaceutical-grade products to date.
BXN said it remained confident in delivering upon its guidance of $20 million revenue for FY2025.
CEO Sam Watson said the quarter marked a significant milestone for BXN and BLS in its journey to becoming Australia’s leading manufacturer and wholesaler of novel medicines.
“The progress our team and business have made toward this objective since the granting of our GMP licence in February 2024 is remarkable,” he said.
“The commercial manufacture of pharmaceutical cannabis pastilles at scale is a unique achievement in Australia.
“We are the leading pastille manufacturer and have established an outstanding THC flower packing service and team.”
Watson said BXN was only at the beginning of its journey to becoming a key player in the global medical cannabis and psychedelics industry.
“Our focus going forward is to build a quality manufacturing business, to serve the entire industry and continue to exceed the expectation of our stakeholders and customers,” he said.
This article was developed in collaboration with Bioxyne, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.