Aussie biotech Actinogen is testing a unique treatment for Alzheimer’s

Pic: Oscar Wong / Moment via Getty Images
An Australian company is approaching a treatment to Alzheimer’s disease from a new direction.
Actinogen (ASX:ACW), led by former Pfizer exec Dr Bill Ketelbey, is in the middle of a Phase 2 trial of its novel Alzheimer’s drug Xanamem.
The working theory, so far born out in animal testing and Phase 1 trials, is that reducing the levels of the stress hormone cortisol in the brains of people with mild Alzheimer’s could treat the symptoms and might even slow down the progress of the disease.

Dementia Australia says 91,000 Australians are diagnosed with dementia, the umbrella category Alzheimer’s falls into, every year. It’s now the leading cause of death for Australian women and second leading cause of death for men, there are only four drugs on the market to deal with it.
Further, these four drugs only treat the symptoms and are only effective for about six months, as they basically squeeze a little bit more run-time out of a progressively deteriorating brain.
In Australia this year dementia is expected to cost the country over $15 billion. By 2056 that rises to $36.8 billion a year. Worldwide, in 2015, the cost of dementia was a whopping $US818 billion.
The opportunity for the company with the next drug to slow or better treat the symptoms therefore is huge, and that is what Actinogen wants to tap into.
The research to find a cure or better treatment, is largely focused on two areas — the build-up of abnormal amyloid and tau proteins, a main cause of Alzheimer’s disease.
“Unfortunately while tens of billions of dollars have been spent on developing drugs to treat amyloid and tau , no one has been successful,” Dr Ketelbey told Stockhead.
“New approaches are needed in the treatment of Alzheimer’s disease and while there are a number of drugs under development and in human trials we’re the only ones targeting cortisol.”
And he should know: he was involved in the development of Pfizer’s Aricept, one of only four drugs used to improve the cognition of people with Alzheimer’s.
On track for trial
Actinogen’s double blind, randomised XanDAu Phase 2 trial of Xanamem in mild Alzheimer’s disease started in May last year and is being conducted at 20 research sites in Australia, Britain and the United States.
It’s the largest trial of this kind to be undertaken to date, by an Australian biotech.
Actinogen is looking to enrol 174 patients and have enrolled 71 so far. They are getting close to the half-way mark in the study.
- Bookmark this link for small cap breaking news
- Discuss small cap news in our Facebook group
- Follow us on Facebook or Twitter
- Subscribe to our daily newsletter
Thirty people have completed the 12-week course and they’re on track to enrol the final patient in the last quarter of calendar 2018.
Full results are expected in Q2 2019.
An interim analysis of the first 50 patients to finish the trial will be done in mid-2018, that may provide early insights into the drug and the study.
Treating the disease
Actinogen was a biotech shell when it licensed Xanamem in 2014 from Edinburgh University.
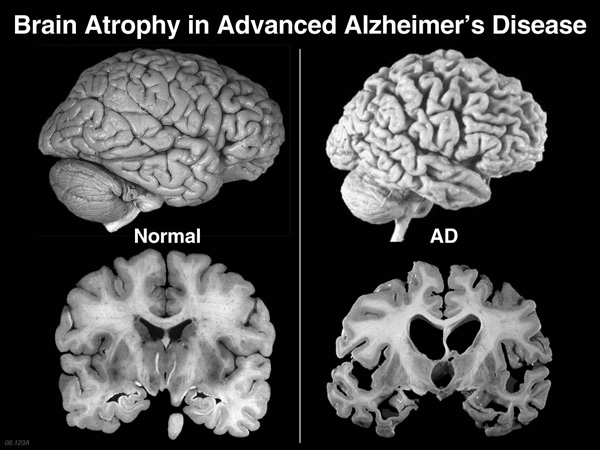
Researchers have discovered that when cortisol levels in the brain were too high over a long period of time it can damage the recent-memory-making part of the brain, the hippocampus.
Until now it’s been very difficult to lower these cortisol levels in the brain, but research at the University of Edinburgh developed Xanamem, a drug that blocks an enzyme in the brain and lowers cortisol.
Actinogen’s research unveiled an additional benefit of Xanamem: animal trials suggested that it could also reduce the amyloid plaque build-up in the brain.
“If we’re able to replicate these animal results in humans, that would represent a very significant advance in the treatment of the disease,” Dr Ketelbey said.
But the biggest problem with treating Alzheimer’s disease is that the damage starts 10-15 years before a person presents with symptoms of Alzheimer’s.
“By the time somebody becomes symptomatic, quite substantial damage has been done to the brain. The goal for Alzheimer’s research is clear, to diagnose as early as possible and to treat as early as possible.
“But at this point in time there is no diagnostic test for identifying who may have the disease or who may have the potential to get the disease sometime in the future.”
Leading the way
Most companies are still focused on the amyloid/tau compounds, but the weight of research is swinging towards new therapies like Actinogen’s.
The company has done a review of the medical literature and over the last few years there has been a dramatic increase in medical publications around cortisol and cognitive decline.
A CSIRO-funded study and another in Nature last year both concluded that raised cortisol levels contributed to cognitive decline and Alzheimer’s in old age.
“The opinion these days is that alternative targets [to research on amyloid and tau compounds] need to be identified, and cortisol is one of those,” Dr Ketelbey said.
“We simply reflect a new era in the development of drugs to treat Alzheimer’s disease and because we started early, in cortisol we’re at the forefront.”
This special report is brought to you by Actinogen.
This advice has been prepared without taking into account your objectives, financial situation or needs. You should, therefore, consider the appropriateness of the advice, in light of your own objectives, financial situation or needs, before acting on the advice.
If this advice relates to the acquisition, or possible acquisition, of a particular financial product, the recipient should obtain a Product Disclosure Statement (PDS) relating to the product and consider the PDS before making any decision about whether to acquire the product.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








