ASX medtechs chasing FDA De Novo for a shot at big US markets

ASX medtechs are taking a shot at big US markets. Pic: Getty Images
- Nanosonics scores FDA De Novo approval in March
- EMVision is also targeting De Novo for its brain scanner
- The company’s big potential in the US
When Nanosonics (ASX:NAN) landed FDA De Novo clearance for its CORIS system back in March, the market took notice, and for good reason.
The CORIS system is not only a world-first device designed to clean the complex inner channels of endoscopes. It also walked the De Novo path – a route the US FDA reserves for genuinely new technologies.
De Novo (which literally means “from new” in Latin) is not a simple clearance. This one is for devices without a predicate – i.e. no similar product already on the market. That means the FDA has to classify and assess the technology from the ground up.
In short: it’s hard to get, and a big deal if you do.
Investors responded in kind, with Nanosonics’ share price jumping by 13% on the news that day.
Other ASX medtechs are also currently chasing De Novo clearance.
PainChek (ASX:PCK), for instance, is waiting on a mid-2025 decision for its pain-assessment app.
TrivarX (ASX:TRI) is also pursuing the De Novo path, with a pivotal trial in the works for its mental health wearable.
Just a few years earlier, in 2016, another Aussie medtech, ProMedicus (ASX:PME), also made inroads in the States, thanks to a key partnership with none other than the prestigious Mayo Clinic.
Since 2016, ProMedicus shares have gone on a jaw-dropping tear, up by more than 9000% today.
Filling the CT and MRI gap
Now, the $145m market capped EMvision Medical Devices (ASX:EMV) is quietly walking a similar path.
We’re not suggesting EMvision is about to pull a Nanosonics or do a ProMedicus victory lap.
But, with De Novo ambitions of its own and an eye on cracking the US, it’s probably one stock worth keeping on the radar.
The Sydney-headquartered company has recently commenced a pivotal trial at Mayo Clinic in Florida, UTHealth in Hourston, as well as Royal Melbourne in Australia.
Mayo Clinic joined the trial after clinicians there reached out to the company, having come across EMVision’s technology.
The Emu product is a portable, bedside brain scanner that aims to help detect strokes and bleeds where access to CT and MRI is limited or simply not practical.
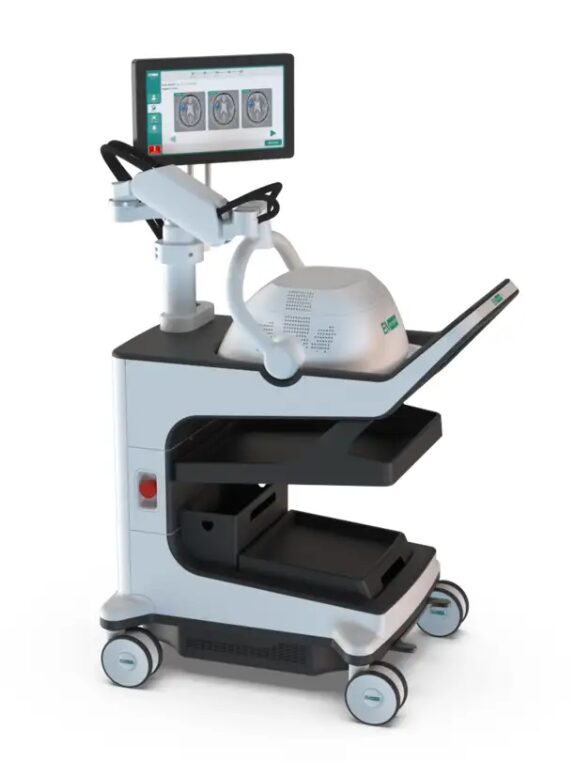
“It has a physical footprint similar to a cart-based ultrasound system, and it’s designed to support point-of-care diagnosis for stroke and stroke-like symptoms,” EMV CEO Scott Kirkland told Stockhead.
The company is also currently chasing De Novo approval for the device.
But if you’re wondering whether this could eventually compete with CT or MRI, the answer is… not really.
And that’s the point.
“We’re not trying to replace a CT or an MRI. In fact, we’re here to fill a gap where they are not available,” said Kirkland.
Emu’s trials and First Responder
Emu isn’t just a ‘me-too’ device – a term for products or drugs that make minor modifications from a better known prototype. It’s going after stroke, one of the biggest health problems on the planet.
One in four adults will suffer a stroke in their lifetime, and most don’t walk away unscathed – two out of three lead to lasting disability.
What makes it worse is that many patients, especially in the regions, can’t get timely access to imaging. The nearest CT could be hours away.
Emu is now in a pivotal trial, aiming to enrol 300 patients across six sites; three up and running, three more to come.
The primary endpoint of the trials is its ability to detect the presence or absence of haemorrhage.
“We have a gold-standard reference diagnosis expert panel that assesses all the ground truths, which are then compared with our diagnostic output to determine our sensitivity and specificity.
“The minimum performance criteria is to exceed 80% sensitivity and 80% specificity. That’s the minimum, but there’s nothing to stop us from going as high as possible.”
Stroke’s not the only target for EMV. One of EMVision’s four trials is also focused on traumatic brain injury.
Then there’s the First Responder.
It’s backpack-sized, battery-powered, and built for use in ambulances and aeromedical units, not hospital wards.

The device has already been tested with the Royal Flying Doctor Service and the Australian Stroke Alliance in outback South Australia.
Next up is a trial inside a Melbourne stroke ambulance, the kind with a CT scanner in the back.
“First Responder doesn’t require a specialist operator, such as a radiographer, and the output can be sent back to a neurologist to guide decision making – whether that’s triage, transfer or potentially treatment at the scene,” Kirkland said.
Mapping the US market
Crucially, EMVision isn’t just taking a punt on the US, it has a clear plan for where its technology belongs.
Kirkland reckons there are around 10,000 Emu opportunities across US stroke wards, ICUs and EDs.
“Within our first targets, there are really two ends of the spectrum.
“One is comprehensive and primary stroke centre, the other are critical access hospitals, which are small regional clinics with 25 beds or less,” he explained.
The First Responder has an even bigger runway – about 60,000 units, covering both road and air ambulances.
And every scan comes with a consumable (a cap and coupling media), adding recurring revenue of around US$25 per scan for Emu, and closer to US$50 for First Responder.
Kirkland is clear-eyed about what comes next.
“We’re targeting to enter the market later in calendar year 2026,” he said.
“It’s a very, very large market. You can do the numbers, and if we can achieve 10% of the market, it is a very large business indeed.”
With De Novo ambitions, a top-shelf US trial, and the Mayo Clinic in its corner, EMVision is playing a long game.
No aggressive claims, just data collection and careful steps.
But in the world of medtech, careful steps in the right direction can sometimes lead to very big leaps.
At Stockhead we tell it like it is. While EMVision is a Stockhead advertiser, it did not sponsor this article.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








