ASX Health Stocks: Rhythm Biosciences scores CE Mark approval, shares jump
Pic: Getty
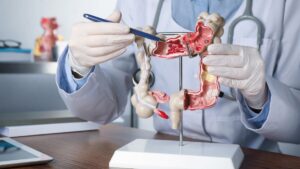
Cancer diagnostics company Rhythm Biosciences Ltd (ASX:RHY) got a regulatory boost this morning, with news that CE Mark approval is now complete for its ColoSTAT blood test platform in the European market.
Shares in the ~$350m company continued their strong 2021 rally with another ~16% gain in morning trade.
ColoSTAT is RHY’s core product, and aims to provide a low-cost, mass market solution for blood tests focused on the early detection of colorectal cancer.
CE (Conformitè Europëenne) Mark approval gives RHY the legal right to sell and advertise the ColoSTAT product in the European market.
Result showed the blood testing platform met European Directives for IVD Medical Devices (98/79/EC), RHY said.
“This critical regulatory achievement is a result of robust and stringent analytical testing and adherence to design and development procedures, as per the Essential Requirements,” the company added.
Documentation for the approval process was managed through Emergo, a global medical device compliance company.
Commenting on the update, Rhythm CEO Glenn Gilbert said the company’s focus will now shift to executing on commercial opportunities in the European market.
Gilbert described the regulatory breakthrough as a “step-change” for Rhythm’s commercialisation strategy.
The company said CE Mark approval gives it access to a European population of around 230 million people, with a total addressable market value of over US$10bn.
“Rhythm is currently assessing its commercialisation options to market ColoSTAT into Europe,” Gilbert said.
In other ASX health news this morning
Biopharmaceutical company StarPharma (ASX:SPL) rose by around 5%, following the release of its CEO presentation at the company’s AGM this morning.
The company provided an update on the commercialisation plans for its product suite utilising dendrimer-based drug delivery, including the DEP drug delivery platform and VIRALEZE, an antiviral nasal spray that’s registered for sale in the UK, Europe and India (but not yet in Autralia).
SPL currently has three DEP products in Phase II trials, with four more at the clinical trials.
While its AGM presentation got a positive response from the market this morning, shares in SPL have fallen from 2021 highs above $2.40 to current levels at around $1.10.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.