ASX Health Stocks: Invion proves photodynamic therapy is effective against the Zika virus

ASX health stocks roundup 7 Sep 2022. Picture Getty
There’s a new development in the fight against the Zika virus.
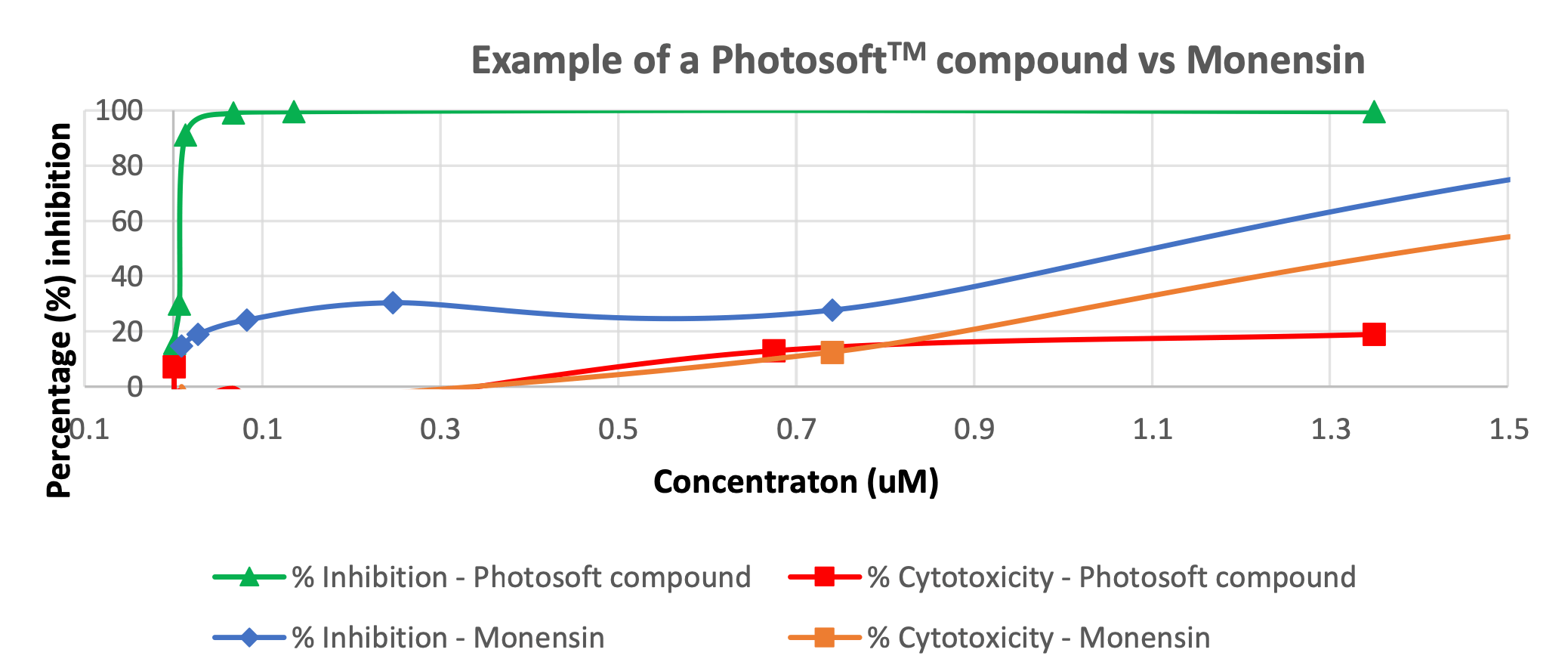
Invion (ASX:IVX) has just demonstrated that its Photosoft compounds were >99% effective in cell-based assays following the application of light.
The study showed that Photosoft was more efficacious than Monensin, an antibiotic which is known to have activity against Zika in previous in-vitro laboratory tests.
The Zika virus is primarily transmitted by the Aedes Aegypti mosquito, is found in 86 countries, and has been linked to birth defects and other neurological complications.
Symptoms associated with Zika include headache, skin rash and joint pain.
A recent report by CSIRO titled “Strengthening Australia’s Pandemic Preparedness” identified the development of novel antivirals as a key science and technology area for strengthening Australia’s pandemic preparedness.
Current estimates put the Zika virus market worldwide at US$16.98 billion ($25 billion) in 2022, and is forecast to grow at a CAGR of 5.4% to 2027.
Invion holds the exclusive rights to the Photosoft (TM) technology in Asia Pacific for the treatment of infectious diseases and atherosclerosis through its agreement with the technology licensor, RMW Cho Group.
The Photosoft technology is a novel next generation Photodynamic Therapy (PDT).
PDT uses non-toxic photosensitisers and light to selectively kill cancer cells and promote an anti-cancer immune response.
Less invasive than surgery and with minimal side effects, PDT is said to offer an alternative treatment option aimed at achieving complete tumour regression and long-lasting remission.
Invion share price today:
Imugene does first patient in Phase 2 trial
Meanwhile, Imugene (ASX:IMU) surged 4.5% this morning after announcing dosing of the first patient in its nextHERIZON Phase 2 clinical trial.
The trial is one of Immugene’s pipelines, investigating the company’s immunotherapy candidate HER-Vaxx in combination with chemotherapy or pembrolizumab in patients with HER-2+ gastric cancer.
The first patient was dosed at the Queen Elizabeth Hospital in Adelaide under the direction of the Principal Investigator Professor, Tim Price.
Imugene expects to open additional study sites in Australia, as well as the US under the FDA investigational new drug (IND) approval received last December.
“Evidence to date has shown the potential to overcome resistance to immunotherapy within GI cancers by increasing cytotoxic and effector immune cells within the tumour microenvironment,” explained Imugene’s CEO, Leslie Chong.
“Immunotherapies such as HER-Vaxx, particularly in combination with immune checkpoint inhibitors such as pembrolizumab, may hold the solution.”
Imugene share price today:
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








