ASX Health Stocks: 4DX skyrockets 54pc after US update on its XV LVAS scanning device

DX surges almost 50pc after an update from the US. Picture Getty
- 4DX surges more than 50pc after an update from the US
- PYC Therapeutic said its fourth drug candidate could be effective in kidney disease
- Pro Medicus has just signed a $20m, eight-year contract in the US
4D Medical up over 50pc
Medtech company, 4DMedical (ASX:4DX), surged 54% this morning after announcing that its XV LVAS scanning device has been included into the U.S. Centers for Medicare & Medicaid Services (CMS).
The CMS is the US federal agency providing health coverage through Medicare, Medicaid, the Children’s Health Insurance Program, and the Health Insurance Marketplace.
Medicare is an important public health insurance scheme for US adults aged 65 years and over; and as of 1 March, there were 65.7 million people enrolled in the program.
The inclusion today means that from 1 January 2024, XV LVAS scans conducted in a US hospital outpatient facility for Medicare patients can be billed to CMS.
CMS has accepted the reimbursement request and finalised assignment of the Category III CPT code for XV LVAS to the rate of US$299 per scan.
4DX’s XV LVAS uses X-rays to create detailed images of lung movement and function during breathing. It gives clinicians a clearer picture of lung health for pulmonary disorders including asthma, COPD, cystic fibrosis, and even cancer.
Also read: 4D Medical’s technology disrupts century-old procedures AND makes over 90pc profit margin
4DMedical CEO and founder, Andreas Fouras, said achieving Medicare reimbursement of US$299 per scan is a major milestone in the company’s progress to secure reimbursement across the entire US healthcare system.
“This ruling will lead to accelerated uptake of XV LVAS, which in turn will support our pursuit of a Category I CPT code,” said Fouras, referring to US coding for different procedures performed in inpatient and outpatient offices and hospitals.
PYC’s fourth drug candidate is effective in kidney disease
RNA-therapies focused biotech, PYC Therapeutics (ASX:PYC), said its fourth drug candidate had disease-modifying potential in patients with end-stage renal failure due to PKD (polycystic kidney disease).
A study conducted by PYC in human 3-dimensional models showed that its investigational drug candidate, PYC-003, could address PKD at the root cause.
Results showed a deduction in cyst size and frequency following treatment with PYC-003 in a human 3D model, which was generated using tissue collected directly from the kidneys of PKD patients.
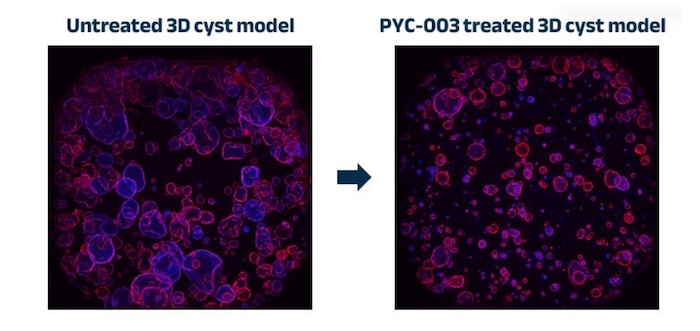
These results complement data from animal models highlighting the ability of PYC-003 to reach the cells affected in PKD.
PKD is a life-changing disease affecting 1 in every 1,000 people, with an addressable market of >US$10 billion per annum. As such, it is an area of major commercial interest for the drug development industry.
Half of the patient population with PKD will require a kidney transplant by the age of 60 due to the absence of current impactful treatment options in this disease.
Following today’s results, PYC now plans to accelerate PYC-003 into human trials.
PYC says an Investigational New Drug (IND) application to the US FDA will enable the commencement of human trials for this drug candidate in the second half of 2024.
Pro Medicus signs $20 million, eight-year deal in the US
Healthcare imaging software company, Pro Medicus (ASX:PME), has just signed a $20 million, eight-year contract with Oregon Health & Science University (OHSU), the preeminent academic medical center in the state of Oregon, US.
Under the agreement, PME’s Visage platform will replace the legacy PACS and vendor neutral archive (VNA)systems throughout the OHSU enterprise.
The contract is for the “full stack” – Visage 7 Viewer, Visage 7 Open Archive and Visage 7 Workflow – to be implemented in the cloud.
PME says the deal represents another step in PME’s rapid expansion into the North American academic medical centre market.
Share prices today:
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








