Actinogen on track with Alzheimer’s trial as research falls into line
Actinogen’s patient recruitment for a landmark Alzheimer’s disease trial is continuing apace and on schedule — just as the research tide is turning to support its approach to treating the disease.
The company (ASX:ACW) has enrolled 74 patients in XanDAu, their Phase 2 trial of Xanamem in Alzheimer’s disease.
That number represents 43 per cent of the total enrolment of 174 patients across 20 research sites in Australia, Britain and the United States.
More than 30 patients have completed the 12-week trial since May last year and full results are expected in Q2 2019.
It is hoped that the drug will treat the symptoms of Alzheimer’s as well as slow down the progression of the disease.
High stress has long term effects
The study has almost reached its half way point as the weight of research swings towards new therapies like Actinogen’s.
Two studies, published in Nature and Psychoneuroendocrinology, indicate that exposure to a period of stress in midlife results in cognitive decline in old age.
- Bookmark this link for small cap breaking news
- Discuss small cap news in our Facebook group
- Follow us on Facebook or Twitter
- Subscribe to our daily newsletter
“This study adds to the growing evidence that raised cortisol, even in mid-life, is toxic to the brain especially with aging,” said Prof Jonathan Seckl from the University of Edinburgh, one of the inventors of Xanamem and an author of the Psychoneuroendocrinology paper.
“It highlights the importance of developing effective treatments that decrease the impact of excess cortisol on the brain as a potential way of slowing the progression of Alzheimer’s disease.
“We have demonstrated that reducing stress hormone in the brain works to restore memory in ageing animals. We are all eagerly awaiting the results from XanADu to see if a similar benefit can be demonstrated with Xanamem in humans.”
The Alzheimer’s problem is growing, fast
Dementia Australia says 91,000 Australians are diagnosed with dementia, the umbrella category Alzheimer’s falls into, every year. Alzheimer’s disease is now the leading cause of death for Australian women and second leading cause of death for men, and there are only four drugs on the market to treat it.

Further, these four drugs only treat the symptoms and are only effective for about six months. They basically squeeze a little bit more run-time out of a progressively deteriorating brain.
In Australia this year dementia is expected to cost the country over $15 billion. By 2056 that rises to $36.8 billion a year. Worldwide, in 2015, the cost of dementia was a whopping $US818 billion.
The opportunity for a company with the next drug to treat the disease is huge, and that is what Actinogen wants to tap into.
The research to find a new treatment, has been largely focused in two areas — the build-up of abnormal amyloid and tau proteins in the brain, one of the hallmarks of Alzheimer’s disease.
“Unfortunately while tens of billions of dollars have been spent on developing drugs to treat amyloid and tau, no one has been successful,” Actinogen CEO Dr Bill Ketelbey told Stockhead
“New approaches are urgently needed in the treatment of Alzheimer’s disease and while there are a number of drugs under development and in human trials, we’re the only ones targeting cortisol.”
And he should know about the medical need: he was involved in the development of Pfizer’s Aricept more than 20 years ago, and while Aricept is the market leader, it is one of only four drugs available to treat people with Alzheimer’s.
Treating the disease
Actinogen, as a listed biotech, licensed Xanamem in 2014 from Edinburgh University.
Researchers there discovered that when cortisol, the “stress hormone”, was raised in the brain over a long time it could damage the recent-memory-making part of the brain, the hippocampus.
Until now it’s been very difficult to lower these cortisol levels in the brain, but researchers at the University of Edinburgh developed Xanamem, which blocks an enzyme in the brain and lowers cortisol.
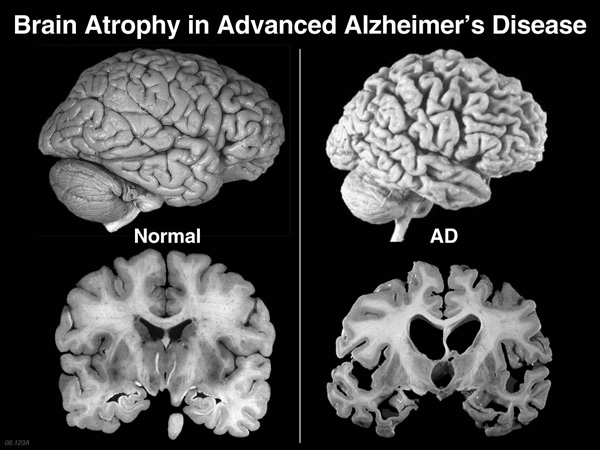
Actinogen’s research unveiled an additional benefit of Xanamem: animal trials suggested that it could also reduce the amyloid plaque build-up in the brain.
“If we’re able to replicate these animal results with cortisol and amyloid, in humans, it would represent one of the most significant advances in the treatment of the disease, in decades,” Dr Ketelbey said.
But the biggest problem with treating Alzheimer’s disease is that the damage starts 10-15 years before a person presents with symptoms of Alzheimer’s.
“By the time somebody becomes symptomatic, quite substantial damage has been done to the brain. The goal for Alzheimer’s research is clear, to diagnose and treat the disease as early as possible.
This special report is brought to you by Actinogen.
This advice has been prepared without taking into account your objectives, financial situation or needs. You should, therefore, consider the appropriateness of the advice, in light of your own objectives, financial situation or needs, before acting on the advice.
If this advice relates to the acquisition, or possible acquisition, of a particular financial product, the recipient should obtain a Product Disclosure Statement (PDS) relating to the product and consider the PDS before making any decision about whether to acquire the product.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








