Actinogen hopes Alzheimer’s trial can beat global 99.6pc failure rate

Pic: sinology / Moment via Getty Images
The sobering news about Alzheimer’s disease is that while it’s emerging as the world’s number one killer disease, there is no drug that cures or alleviates the ailment.
That’s not for wont of trying, either.
The US government’s clinical trials database lists 1810 Alzheimer’s related trials. Of these, 336 are actively recruiting, 967 have been completed and 137 terminated (some with extreme prejudice).
The trials include a heroic but ultimately forgettable attempt to evaluate the therapeutic effect of coconut oil.
All up, the failure rate is 99.6 per cent, with only 10 to 27 per cent of patients trial-eligible in the first place.
Joining the list of those seeking to crack a cure is Actinogen Medical (ASX:ACW), which is carrying out the biggest Alzheimer’s dementia study ever done by an Aussie biotech.
Actinogen chief Dr Bill Ketelbey was involved in developing Aricept, which remains the leading Alzheimer’s treatment despite being developed 25 years ago.
Chairman Dr Geoff Brooke is well known as the founder of venture capital firms Medvest and GBS Venture Partners.
In December the company’s petite board was bolstered with the inclusion of Dr George Morstyn, former chief medical officer at US pharma Amgen.
And for a company whose technology evolved from the Edinburgh University, what better time for us to visit Actinogen just after January 25?
For proud Scots, it’s not Australia Day/Invasion Day Eve but Burns Day, the Scots’ day of celebration of poet laureate and Auld Lang Sine creator Robert Burns.
- Bookmark this link for small cap breaking news
- Discuss small cap news in our Facebook group
- Follow us on Facebook or Twitter
- Subscribe to our daily newsletter
Scary trends
Alzheimer’s is already the leading cause of death for women in Australia and second overall behind heart disease.
Within five years Alzheimer’s will be the leading cause of death, partly because of progress with preventing heart disease and killer cancers.
Globally there are 50 million people with Alzheimer’s disease and the number is expected to double every 20 years.
Almost one-third of 85-year olds have the disease, although in the past we used to think gran and pop were just a bit senile.
“Everyone’s now looking to novel approaches for treating the disease and that’s where Actinogen comes to the fore,” Dr Ketelbey says, adding that any developed drug is likely to be used in combination with existing drugs.
Actinogen’s approach
Actinogen’s work revolves around the novel drug candidate Xanamem, which is designed to inhibit production of the naturally occurring stress hormone cortisol in the brain.
A growing screed of research links excessive cortisol with the development and progression of Alzheimer’s.
Xanamem aims to negotiate the blood-brain barrier — something other drugs can’t do properly — and thus deliver the active ingredient more effectively.
In short, Xanamem has the potential to modify the disease, which no other drug has managed.
“If we can demonstrate [that] in our trials, we have a world-beating product beyond a doubt,” Dr Ketelbey says.
(Like everything in biotech trials, note the use of the word “if”.)
Recruiting now
In May 2017, Actinogen started recruiting for its headline clinical trial, called Xanadu.
This has nothing to do with one of Olivia Newton John’s forgettable recording efforts, Kublai Khan or even Orson Welles, but is a 174-patient phase II trial for mild Alzheimer’s sufferers.
Xanadu is constructed as a double-blinded, 12-week, randomised, placebo-controlled study. Previous animal models showed “significant cognitive improvement” with Xanamem after 28 days.
The trial will enrol 174 patients across 20 research sites here and in the US and the UK. To date, 66 patients have been recruited and in early September the first patient completed a 12-week treatment, plus four-week follow up.
Top-line results are due in early 2019, which sounds like a long way away, but time will fly.
Dr Kitelbey said by June this year the independent data safety monitoring board would review the unblinded data from the first 50 of the 156 evaluable patients.
But as this data will be blinded to the company, there will be no opportunity for a statistical analysis.
To gauge efficacy, patients will be subjected to a number of widely used cognitive tests, such as the mini mental state examination (MMSE) and the ADAS-Cog14.
For example, patients might be asked to remember three unrelated terms such as ‘apple’, ‘boat’ and ‘chair’. They are then guided to another topic and asked to recall the words a few minutes later.
Such measures aren’t exactly perfect, because on a bad day anyone could struggle to remember their chairs and their pears (or apples).
But given the absence of biomarkers — signals in the body to flag the presence of a disease — such tests will have to suffice.
Fully-funded
The Xanamem trial is fully-funded after the company raised $5.28 million in an oversubscribed placement in December.
At a general meeting on January 18, shareholders voted ‘aye’ to the two-tranche placement, done at 4c (then a 22 per cent discount) and accompanied by a one-for-two options issue exercisable at 6c a share.
Should Actinogen raise more moolah — and never say never — the company enjoys the support of a phalanx of local investors including Perth’s Forrest Capital, Sunset Capital and Tony Grist (Perth man about town and co-founder of Amcom Telecommunications).
Xanadu and beyond
Xanamem’s opportunities lie with diabetes-related cognitive impairment, post-traumatic stress disorder, Parkinson’s disease, epilepsy, schizophrenia and post myocardial infarction.
All of these are related to elevated levels of the cortisol “stress hormone”.
The Uni of Edinburgh, Actinogen’s biggest holder, is interested in substantially funding diabetes and cognitive impairment trials, so never accuse Scots of being tight with their money.
It’s possible that Actinogen would go it alone on a lengthy phase III Alzheimer’s trial — a high-risk, high-reward route.
Dr Boreham’s diagnosis:
Dr Ketelbey reckons that, globally, a phase II Alzheimer’s triallist attracts an average market valuation of $US800 million ($992 million).
Given Actinogen’s lowly $30 million market cap, he contends the company is undervalued.
Then again, everyone thinks their own baby is cuter than the one in the crib next door.
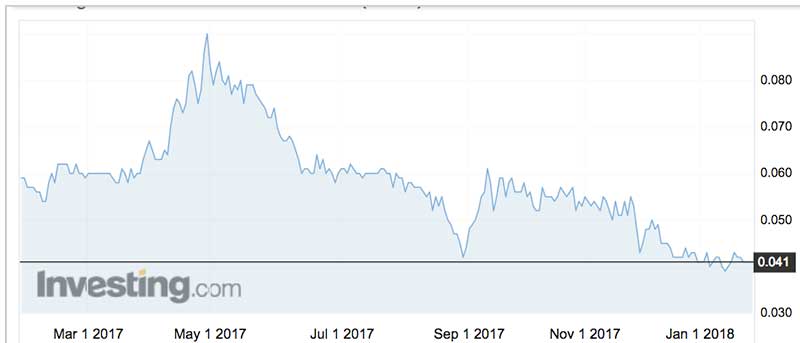
Actinogen shares peaked at 15c in April 2015 and then made an assault on the 9c level in April last year.
Post-placement, the stock is now trading at 14-month lows.
Dr Ketelbey reckons the Xanadu trial is not a binary outcome because of Xanamem’s other potential applications.
Still, Alzheimer’s is clearly Actinogen’s main focus and last year’s demolition of Living Cell, Innate Therapeutics and Resapp shares show how unforgiving investors are in the wake of failed clinical trial results.
So let’s drink a cup of kindness to the boffins from Actinogen (and Edinburgh) as they strive to cure this cruel disorder.
This article first appeared in Biotech Daily
Disclosure: Dr Boreham is not a qualified medical practitioner and does not possess a doctorate of any sort. He is also wondering why he put his car keys in the fridge.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions. The content of this article was not selected, modified or otherwise controlled by Stockhead. Stockhead has not provided, endorsed or otherwise assumed responsibility for any financial product advice contained in this article.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








