A year after cold shower Acrux rebuild gets underway with first generic drug

Pic: Godji10 / iStock / Getty Images Plus via Getty Images
It’s coming up to a year since the cold shower that shook up drug maker Acrux, forcing it to launch a push into the generic drugs market after the US shut the door on its flagship testosterone product.
Regulatory changes due to concerns of heightened health risk from testosterone prompted US drug major Eli Lily to stop distributing the Acrux product after it had earlier faced mounting pressure from rival producers.
Ahead of the Eli Lily move, Acrux took protective action, worried that any further erosion of sales in the US could undermine the company’s financial future.
In mid-2015 Acrux outlined its plan to push into generics – low cost versions of drugs which are launched once existing drugs lose their patent protection.
And two years ago it said it would lodge its first application with the gatekeeper to the US drugs market, the Food and Drugs Administration, by mid-2018.
This week, Acrux confirmed it had lodged its first new drug application with the FDA for a generic version of Jublia, an antifungal agent with annual revenues of around $US280 million.
Acrux got the paperwork in on the first possible day after the drug came off patent, which puts it in prime position to snatch around half of all sales of the drug.
Typically, once a generic drug is launched, the price declines by around 40-50 per cent — and the first generic product is snapped up by wholesalers all but guaranteeing it a big slice of the market.
First to file
“Acrux was the first to file,” chief executive Michael Kotsanis told Stockhead.
Although whether it will be the first challenger to hit the market is unclear since it will take time for the FDA to consider the applications received and give them the greenlight to enter the market.
For the first generic in this market, the sales potential is likely to be close to $100 million — although how competitors will react to the looming changes will take time to become clear.
“Typically, generics take around half the market,” Mr Kotsanis said.
“Generics are driven by a few large wholesaler/distributors. A generic typically takes half of the market in the first few weeks after launch.”
Aware of the potential value of getting its generics program up and running, Morningstar in mid-July raised its estimate of the fair value of Acrux shares to 15c from 13c due to the imminent lodging of the FDA application.
Generic drugs in the pipeline
Acrux has as many as 13 generic drugs in the pipeline — with launch prospective by the end of 2019 — which will go some way towards restoring investor value in the company.
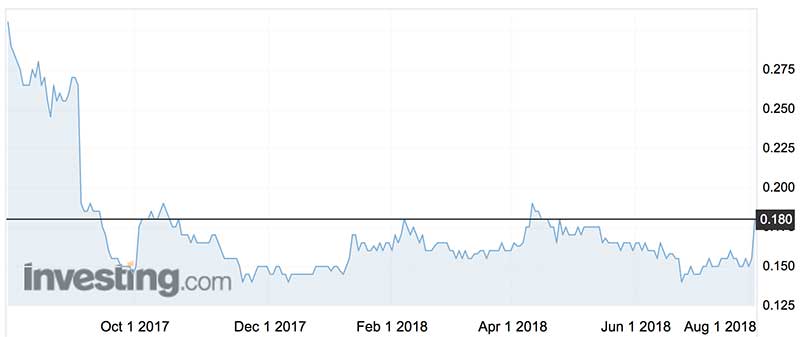
At its peak, Acrux shares topped $4 five years ago, but the steady flow of bad news as the future of its testosterone agent faded saw the shares fall to around 14.5c. They rallied on the latest news, trading Thursday at
“There are 13 products in the pipeline and additional applications are planned by year-end,” Mr Kotsanis said. “The drugs will start to come out in a steady stream.”
- Subscribe to our daily newsletter
- Bookmark this link for small cap news
- Join our small cap Facebook group
- Follow us on Facebook or Twitter
An indication of the potential worth of drugs in the US generics market was given recently with the move by Mayne Pharma to pay up to $US30 million for a generic with only a modest share of a market worth around $US66 million.
Unlike Acrux, Mayne can market the drug it is acquiring through its own sales team, helping to maximise margins whereas Acrux will use partners to handle the production and also marketing.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








