ASX-listed Zelira is going head-to-head with Big Pharma. We spoke to its CEO about its place in a US$62 billion market

Zelira’s ZLT-L-007 shows better results than Pfizer’s Lyrica. Picture Getty
- Zelira announced that its diabetic nerve pain drug outperforms Pfizer’s Lyrica
- Diabetes is a huge market, estimated at a massive US$62 billion in 2022
- Stockhead reached out to Zelira’s CEO, Dr. Oludare Odumosu
Diabetes is one of the leading causes of deaths in the world, ranking number 9 on the World Health Organisations’s list.
According to data from Precedence Research, the global diabetes drug market size was estimated at a massive US$62 billion in 2022, and is projected to double by 2032.
Until today, scientists have not yet found a cure for the disease, but what they do know is that high blood sugar causes nerve damage, resulting in mild numbness in the legs and sometimes pain that can be quite disabling.
As many as 50% of people with diabetes suffer from nerve pain, and half of all amputations annually are associated with the disease.
For years, the standard drug on the market to deal with the pain has been Pfizer’s pregabalin, sold under the brand name Lyrica. This drug generates around US$5 billion a year in annual sales.
But last month, the medical world was rocked when ASX-listed Zelira Therapeutics (ASX:ZLD) said it had come up with an alternative drug that could perform better than Lyrica.
The ZLD share price tripled when the company announced that top-line results for its proprietary ZLT-L-007 drug had achieved better reduction in NRS pain scores than Lyrica.
The study showed that ZLT-L-007, a cannabinoid-based oral capsule, was safe and well-tolerated, meeting the primary endpoint for safety with no Serious Adverse Events (SAE).
The study also met its secondary endpoints, including significant decreases in Visual Analog Scale (VAS) and Short-form McGill scores, among others.
A closer look at Zelira’s study
These compelling outcomes have provided confidence for Zelira to evaluate further progression of ZLT-L-007 into formal FDA clinical trials.
Zelira’s CEO, Dr. Oludare Odumosu, told Stockhead that he was excited about the huge potential for ZLT-L-007 to not only generate revenues, but also impact real-life debilitating conditions in patients worldwide.
“The level of pain relief that Lyrica provided was economically exciting over the past few years, and that drug generated something around US$5 billion a year in annual sales,” said Odumosu.
“Which means that if Lyrica, with the level of pain relief and side effect profile that it had, could be that successful, we’re extremely excited about Zelira’s prospects.”
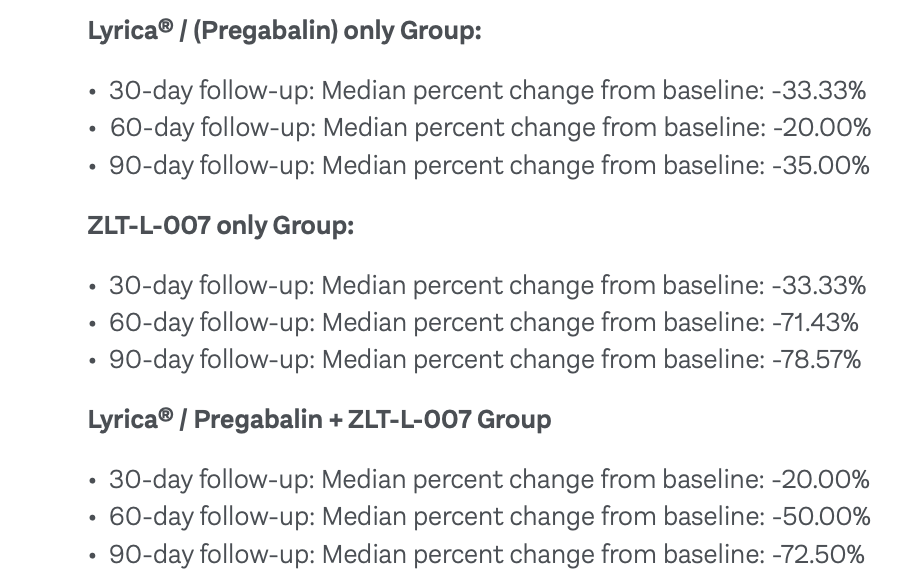
The ZLT-L-007 trial was designed as an observational multi-arm, head-to-head study with 60 subjects, powered to show statistical difference with approximately 20 patients in each Group.
In Group 1, there were 22 patients already taking Lyrica at the prescribed dose as recommended by their doctor.
In Group 2, a total of 18 patients took one capsule of Zelira’s ZLT-L-007 by mouth twice daily.
And in Group 3, the trial studied 20 patients who are already taking the Lyrica drug at the prescribed dose, but they also received one ZLT-L-007 capsule by mouth twice daily.
The results were pretty convincing for the Group 2 Cohort (ZLT-L-007 only), with the most substantial reduction in pain severity happening particularly at the 60-day and 90-day follow-up periods.
Odumosu explained that the study was designed a bit differently in that ZLT-L-007 wasn’t compared against placebo, but was instead pitted head-to-head against the current gold standard, Lyrica.
“Normally, you would do a phase one study in healthy volunteers, but we had an opportunity to seek an IRB (Institutional Review Board) approval that allowed us to design the study more aggressively in that it was tested against the gold standard.”
The positive results also represent a thumbs-up to Zelira’s patent-protected technology, Zyraydi, which was utilised to create the free-flowing powder formulation used for ZLT-L-007.
The Zyraydi technology enabled ZLT-L-007 to be delivered in an easy-to-swallow, relatively small pharmaceutical-grade size 2 capsule.
‘Multiple shots on goal’
Odumosu said this study is sufficiently closed and the company will now proceed to additional validation.
The next step would be for the Zelira team to go and put on the board a project plan towards full FDA trials.
“We would want to look at the results completely, fully analysed, which will take a few months, and report that in a formal report,” Odumosu explained.
“Probably a phase 2 design would be the natural next point for a study like this, and that would take some planning.
“We would hope that within the next three or so years, we are having a different conversation about the potential of this product towards formal trials.
“There’s a real value here, and our company is dedicated to putting the right resources behind driving this forward,” Odumosu told Stockhead.

Apart from ZLT-L-007, Zelira has other CBD programs already in the market, including a tincture blended with pharmaceutical-grade refined, bleached and deodorised olive oil called HOPE 1.
HOPE 1 is is a 1:1 THC:CBD ratio with 5mg/mL of THC, 5mg/mL of CBD, and is indicated for patients with Autism Spectrum Disorder.
The company’s ZENIVOL meanwhile is an oil-based formulation or powder capsule, again utilising the Zyraydi technology, for patients with chronic insomnia.
“All of these things sit within our multiple shots on goal strategy, which are multiple value points the company has created.
“And now we’re starting to see the results of those multiple shots across multiple products,” said Odumosu.
Other ASX cannabis stocks in a similar space
Incannex is developing medicinal cannabis pharmaceutical products and psychedelic medicine therapies for multiple indications.
These include treatment of obstructive sleep apnoea (OSA), traumatic brain injury (TBI) and concussion, lung inflammation (ARDS, COPD, asthma, bronchitis), rheumatoid arthritis, inflammatory bowel disease, anxiety disorders, addiction disorders, and pain, among others.
The company’s anti-inflammatory drug candidate IHL-675A was recently found to be well-tolerated, with no adverse events of concern and no serious adverse events in a Phase 1 study.
Meanwhile, Incannex’s Phase-2/3 clinical trial investigating IHL-42X cannabinoid treatment of obstructive sleep apnoea (OSA) is also progressing well.
Elsewhere, the company’s psychedelics focused subsidiary, Psychennex, is set to open multiple psychedelic clinics in Australia to capture the emerging opportunities in psychedelic-assisted psychotherapy.
Neurotech International (ASX:NTI)
Neurotech focuses predominately on paediatric neurological disorders, where there is persistent neuro-inflammation.
The company has completed a Phase I/II clinical trial in Autism Spectrum Disorder (ASD) with NTI164, and is also conducting other studies including Phase I/II trials in Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS) and Rett Syndrome.
The company recently presented at the 2023 International Rett Syndrome Foundation meeting in Nashville titled: “NTI164: A Novel, Full-Spectrum Medicinal Cannabis-Derived Treatment for Rett Syndrome”.
NTI164 is a proprietary drug formulation derived from a unique cannabis strain with low THC (M<0.3%), and a novel combination of cannabinoids including CBDA, CBC, CBDP, CBDB and CBN.
Zelira and other share prices today:
The views, information, or opinions expressed in the interview in this article are solely those of the interviewee and do not represent the views of Stockhead.
Stockhead has not provided, endorsed or otherwise assumed responsibility for any financial product advice contained in this article.
At Stockhead we tell it like it is. While Incannex Healthcare and Neurotech are Stockhead advertisers, they did not sponsor this article.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








