PharmAust’s MPL drug shows anti-cancer effects across multiple cancer types
Health & Biotech
Health & Biotech
Research from the Olivia Newton-John Cancer Research Institute on PharmAust’s Monepantel (MPL) anti-cancer drug has been published in the Wiley peer review journal – highlighting that the drug has anti-cancer effects across multiple cancer types.
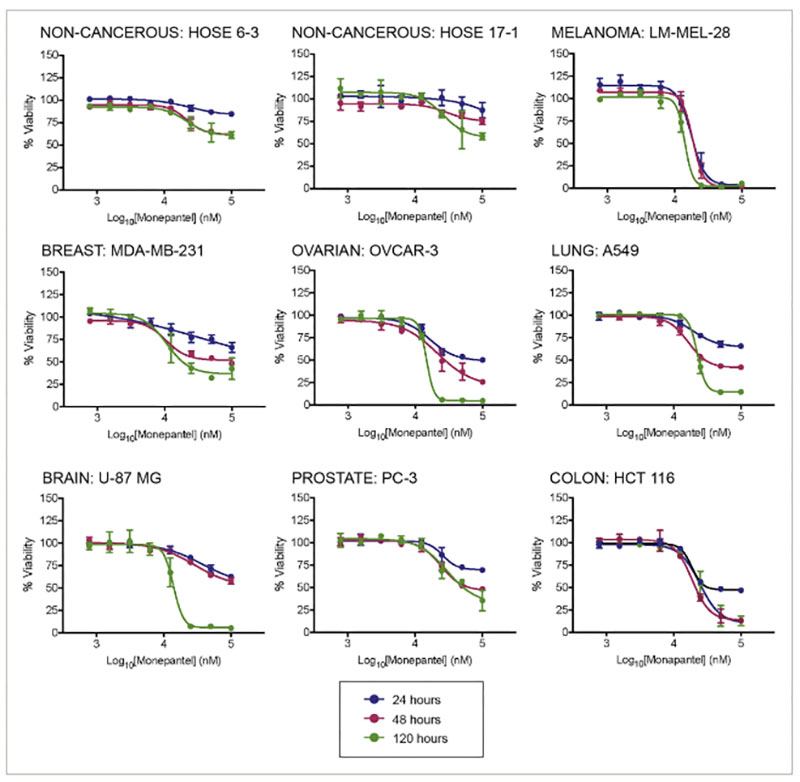
The research shows that Monepantel has broad anti-cancer effects across multiple cancer types including melanoma, lung, breast, brain, colorectal, prostate, and ovarian.
In some cases, this happens through apoptosis, where the cells die off, but, even when apoptosis doesn’t happen, MPL can still stop the cells from reproducing by disrupting the cell cycle.
It’s kind of a big deal for the biotechnology company, with the treatment well-tolerated in humans and dogs, leaving PharmAust (ASX:PAA) uniquely positioned to commercialise MPL for treatment of human and veterinary cancers as well as neurodegenerative disease as it advances a reformulated version of this drug through Phase 1 and 2 clinical trials.
PharmAust executive chairman Dr Roger Aston said “this analysis of the mechanisms of action of MPL in conjunction with its very low toxicity offers a potential new paradigm in the regulation and management of cancer.”
When the researchers analysed the genes in the cancer cells treated with MPL they found that many of the genes involved in cell division were turned off, while genes involved in the stress response for apoptosis were turned on.
As these outcomes are associated with mTOR signalling, cell cycle and autophagy, the researchers believe this is the likely MPL anti-cancer trigger mechanism.
Other studies have suggested that MPL can also cause a different kind of cell death called autophagy, but this research identified that autophagy isn’t necessary for the drug to be effective.
Just last week the company received commitments for a placement to raise $2.5m at 8c per share to fund the preparation for upcoming human trials, further manufacture of additional MPL tablets for human and canine trials and to strengthen working capital.
The drug is also a promising candidate for Motor Neurone Disease/Amyotrophic Lateral Sclerosis (MND/ALS).
Interim results are expected later this month for the Phase 1/2 Human MND trial currently underway, the initial initial safety & PK data from which will fast track a Phase 2 human anti-cancer trial. PharmAust previously announced a Principal Investigator had been identified in the US to conduct these trials.
The Phase 2 vet cancer trial is expected to be completed mid 2023 with assay results from 10 x plasma samples expected later this month.
PharmAust is also targeting a corporate outcome this calendar year on the licensing or sale of MPL’s vet cancer applications following commercially valuable Phase 2 outcomes.
This article was developed in collaboration with PharmAust Limited, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.