You might be interested in
News
Closing Bell: ASX slips from record highs, but Black Friday sales could be catalyst for next week
News
ASX Small Caps Lunch Wrap: CPI data put a rocket up the ASX, but REX is circling the drain
Health & Biotech
Health & Biotech
Living Cell Technologies says its Parkinson’s phase 2b clinical trial has produced some mixed results — the brain implant worked for some but not for others.
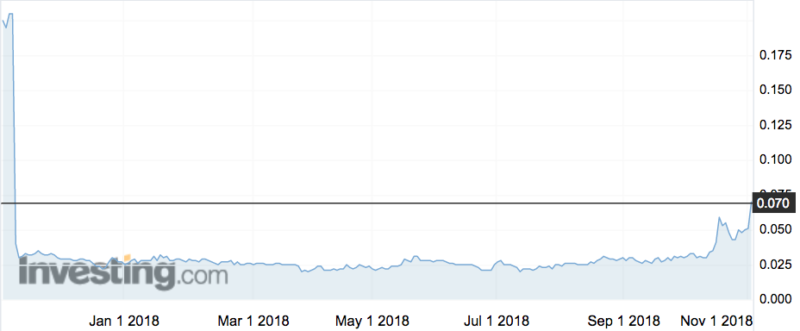
Investors responded well to the news, sending the stock up 47 per cent to an intraday high of 7.5c.
The biotech (ASX:LCT) is trying to fight Parkinson’s disease with a drug that is implanted into the brain.
The initial analysis of data from 18 months of follow-ups showed “statistically significant improvement” in patients who received 80 microcapsules of the drug dubbed NTCELL.
But there was no benefit observed in patients who had 120 capsules implanted. In fact, there was evidence of inflammation “which may have compromised efficacy in this group”.
Company chief Dr Ken Taylor said the company would be discontinuing the 120 capsule dosage.
“That was our maximum dose, and it’s still safe, but it overflowed the space in the brain which caused inflammation in some patients,” he told Stockhead this morning.
“That could have impacted the response to the treatment. Inflammation reduces, so the patients are okay, but we won’t be progressing the 120 capsule dosage any further.”
Dr Taylor said the company was not surprised to see better results at the 18-month follow-up than they did after six months, when its shares collapsed 85 per cent when the company reported “no statistical difference” between the efficacy of NTCELL relative to the control group, in its 18-patient phase 2b trial.
“Patients who received the 40 and 80 capsule dose are showing statistically significant improvements in Parkinson’s symptoms compared to baseline,” he said.
“The original six-month endpoint was too early so it wasn’t showing signs at six months. Which makes sense, as the cells don’t grow quickly.”
Living Cell’s drug NTCELL works by coating choroid plexus cells from newborn pigs — the cells that produce cerebrospinal fluid in the brain — and turning that into a capsule that can be implanted into the brain.
Clinical trials are generally divided into three phases. Phase 1 focuses on safety, phase 2 tests effectiveness and Phase 3 examines whether it’s an improvement on existing treatments. Sometimes phases are divided into stages A and B, where B is generally more rigorous.
Living Cell shares jumped 50 per cent back in May when the company showed some improvement from a 12-month follow-up.
The next steps for NTCELL are a two-year follow-up for these patients in May, which also coincides with the four-year follow-up for patients in its Phase I and 2a trial for the same condition and treatment.