Avita gathers $16.9m war chest to commercialise its ‘spray-on skin’
Health & Biotech
Health & Biotech
Avita Medical has announced a $16.9 million capital raising in a push to commercialise its ‘spray-on skin’ products in the US.
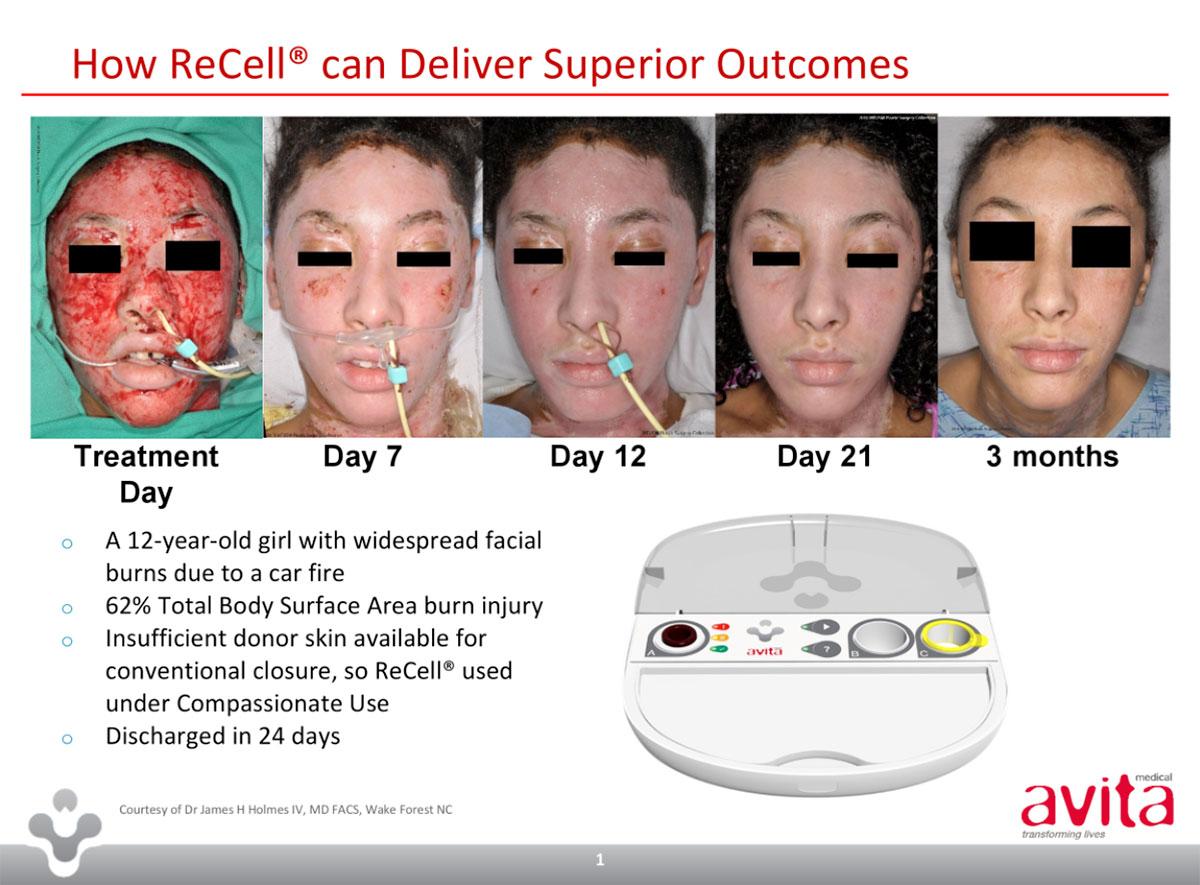
Avita (ASX:AVH), which specialises in the treatment of wounds and skin defects, is trying to commercialise a spray-on skin technology called ReCell developed by former Australian of the Year Fiona Woods.
A private placement to institutional investors raised $4.5 million at 4.5c. Avita is now looking for another $12.4 million in a rights issue to existing shareholders at the same price.
Avita shares fell 24 per cent to 6.5c after the announcement on Wednesday.
About $9.75 million would fund the initial stage of a $US24.3 million research contract to look into pediatric burns care with the US health department’s Biomedical Advanced Research and Development Authority (BARDA).
The capital raising comes as Avita submitted pre-market approval for its ReCell device last month.
“This fund raising is expected to underpin Avita through key milestones including, initial BARDA product procurement, US FDA approval, and the launch of ReCell in the US burns market,” chief executive Mike Perry told investors.
“What we consider compelling for investors is the strength of our clinical data from the two US trials of 131 patients at 12 leading US burn centres demonstrating that use of the ReCell device results in significantly less donor skin harvesting, relative to standard care, for treatment of burn injuries.”
Almost a third of burns in the US occur in patients under 16 years of age and with donor skin at a premium, it’s hoped the study will lead to better results.