ASX suspends Bod after questioning move from cosmetics to cannabis
Health & Biotech
Health & Biotech
The ASX has hit the pause button on Bod Australia, questioning whether the would-be medical marijuana importer might have broken stockmarket rules when shifting from cosmetics to cannabis.
Bod (ASX:BDA) flagged that it was going green back in April, and has been open about its aims to build a medicinal cannabis business and embark on clinical trials.
Bod’s company secretary Andrew Bursill said the ASX was considering whether “medicinal cannabis, as part of its natural medicine portfolio, represent a significant change in the nature of the company’s activities”.
Bod describes itself as a “developer and distributor of natural, evidence-based cosmetics and natural medicines”.
An ASX spokesman said Bod was made aware of potential Chapter 11 implications several months ago, but only now had received submissions from the company.
The ASX’s Chapter 11 rules outline steps companies must take when disclosing a significant change of activity.
“The obligation is with the company to ensure compliance with the rules. We encourage companies to speak with ASX early in the process. Medical cannabis listings, including existing listed companies proposing to move into that space, are on ASX’s radar.”
In October, the ASX said it was receiving an increase in inquiries around medical cannabis businesses entering the market, but many were coming from early stage businesses with little or no operating or financial history.
It also pointed out that medicinal cannabis businesses coming from the US had to be legal there if they wanted to list in Australia.
Import licence on the way
Bod is yet to join the six companies allowed to import medicinal cannabis products into Australia. But it does now have initial licences in place from the NSW Department of Health and the Therapeutic Goods Administration (TGA).
Those approvals will let Bod store and supply medicinal cannabis, and they are now seeking an import licence from the Office of Drug Control (ODC).
The plan is to bring in products and raw materials made by Swiss partner Linnea to sell and to use in clinical trials.
Bod wants to run a nine-month initial clinical trial next year in Victoria to test the safety, tolerability and pharmacokinetics, or movement of drugs through the body, of its cannabinoid formulations.
At the end of September Bod had $2.3 million to spend on these ventures, as well as others including distribution deals for non-cannabis products like skin care ranges and pregnancy supplements.
It managed to grow sales by 188 per cent to $130,000, of which it banked $67,000 in that quarter.
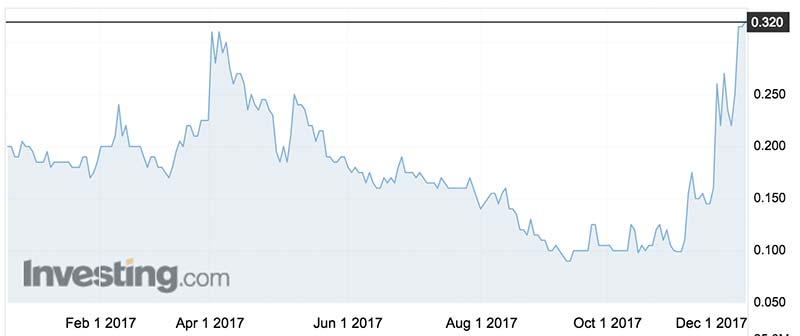
Bod’s shares last traded at 32c — triple its price this time last month — before the current trading halt.
Pot stocks broadly fell on Tuesday, though they have made big gains in recent months.