Breakthrough: Researchers ‘harvest’ carbon dioxide to make valuable products
News
News
Researchers from Oregon State University have found a way to harvest carbon dioxide (CO2) from smokestacks and use it to create commercially valuable chemicals thanks to the development of a novel compound.
The new metal organic framework, loaded with a common industrial chemical – propylene oxide – can catalyse the production of ‘cyclic carbonates’ while scrubbing CO2 from factory flue gases.
Cyclic carbonates have a broad range of industrial applications, including as polar solvents, precursors for polycarbonate materials such as eyeglass lenses and digital discs, electrolytes in lithium batteries, and precursors for pharmaceuticals.
“These are very exciting findings,” OSU College of Science chemistry researcher and study leader Kyriakos Stylianou said.
“And being able to directly use carbon dioxide from impure sources saves the cost and energy of separating it before it can be used to make cyclic carbonates, which will be a boon for the green economy.”
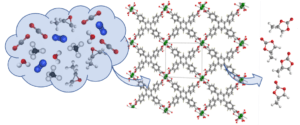
The new, three-dimensional, lanthanide-based metal organic framework – or MOF – can also be used to catalyse cyclic carbonate production from biogas, a mix of carbon dioxide, methane and other gases arising from the decomposition of organic matter.
“We’ve taken a big step toward solving a crucial challenge associated with the hoped-for circular carbon economy by developing an effective catalyst,” Stylianou said.
“A key to that is understanding the molecular interactions between the active sites in MOFs with potentially reactive molecules.”
MOFs can be designed with a variety of components, which determine the MOF’s properties, and Stylianou said lanthanide-based materials are generally stable because of the relatively large size of lanthanide ions.
And that’s true as well with lanthanide MOFs, where the acidic metals form strong bonds with the linkers, keeping the MOFs stable in water and at high temperatures – which is important because flue gases and biogas are hot as well as moisture rich.
The lanthanide MOFs are also selective for carbon dioxide, meaning they’re not bothered by the presence of the other gases contained by industrial emissions and biogas.
“Using our MOFs, stable after multiple cycles of carbon dioxide capture and conversion, we describe the fixation of carbon dioxide into the propylene oxide’s epoxy ring for the production of cyclic carbonates,” Stylianou said.