MGC manufacturing facility gets green light to produce cannabis epilepsy drug
Health & Biotech
Health & Biotech
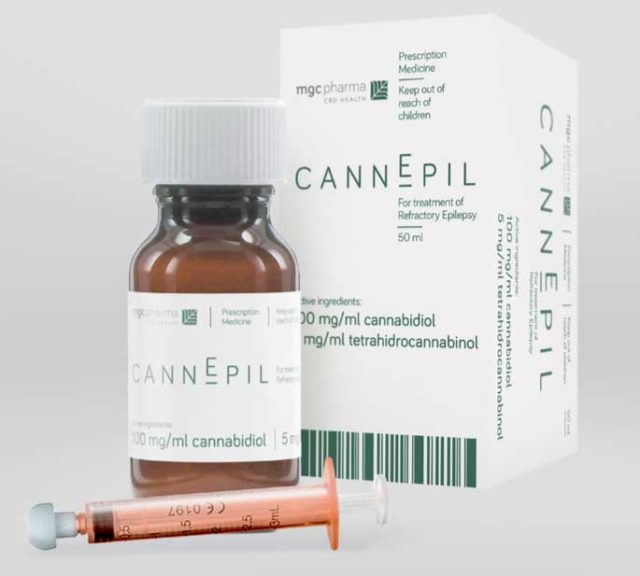
MGC Pharma’s European manufacturing facility has been given the green light, paving the way for the first batch of its medical cannabis-based epilepsy product CannEpil.
This interim licence is one of the final steps in full Good Manufacturing Practice (GMP) certification, a process that has already taken 18 months including formal inspection and subsequent report earlier this month.
All that is left is for MGC Pharma (ASX:MXC) to produce the first batch at the facility and get final approval for validation of manufacturing, analysis and protocols.
CannEpil will be the first product made at the freshly-approved GMP facility, and what the company say will be the first of many other pharmaceutical grade medicinal cannabis products that can and will be made there.
“MGC has a vision of becoming a leading pharmaceutical-grade medicinal cannabis company and the receipt of GMP certification for our European manufacturing facility is a key milestone on this journey,” they told the market.
“Once we receive full certification, we will be positioned to rapidly progress our operations in Europe with a core focus on medical research and development of the Company’s pharmaceutical products pipeline.”
This approval positions MGC Pharma as one of a only a small number of such facilities in Europe and the only one of this grade.
The company signed an agreement with specialist Australian pharmaceutical distributor HL Pharma last year, to bring the products to the Australian market and already approximately 100 patients have registered.
About 25,000 Australians are diagnosed with epilepsy each year and some 240,000 are now living with the disease.
CannEpil is aimed at drug-resistant or refractory epilepsy – which covers about 30 per cent of all cases or around 70,000 in Australia, according to EAA.
A 10 per cent share of the potential market of 70,000 Australian adult patients would deliver $70 million in annual turnover for the CannEpil product alone.
CannEpil will be available with an authorised doctor’s prescription for a regular retail price of less than $800 – a price point that is lower than the competing product on the market, MXC says.
This deal comes after the company announced its skincare subsidiary, MGC Derma, would stock its products on UK retailer, Cult Beauty.
Shares spiked to as much as 11.5c on the news, and were trading at 10c on Thursday open.
This special report is brought to you by MGC Pharma.
This advice has been prepared without taking into account your objectives, financial situation or needs. You should, therefore, consider the appropriateness of the advice, in light of your own objectives, financial situation or needs, before acting on the advice.
If this advice relates to the acquisition, or possible acquisition, of a particular financial product, the recipient should obtain a Product Disclosure Statement (PDS) relating to the product and consider the PDS before making any decision about whether to acquire the product.