With various studies identifying exosomes as a preferred drug-delivery option for some mRNA therapeutics, Exopharm is centre stage to be a leader in the field.
You can sense the excitement of Exopharm (ASX:EX1) founder and CEO Dr Ian Dixon with advances made by the company coinciding with heightened interest in mRNA therapeutics.
“The time for exosomes as an essential part of modern medicines is with us,” he said.
The success of mRNA vaccines against Covid-19 has renewed interest in mRNA therapeutics, with potential to change the standard of care for many diseases and advance personalised medicine.
mRNA therapeutics offer the promise of generating therapeutic proteins (gene-products) inside the patient’s body (in situ) potentially benefiting patients with a range of conditions.
Drug-delivery is the main hurdle holding back many potential mRNA therapeutics, and the ideal characteristics for a drug-delivery chassis are explained in a recent scientific paper titled Unlocking the promise of mRNA therapeutics.
Among authors of this paper is co-founder of big pharma Moderna, now a US $63 billion market cap company, Dr Kenneth Chein. Moderna has pioneered development of mRNA vaccines during the covid pandemic.
The expert says…
As Chien’s paper explains the drug-delivery requirements for mRNA vaccines and mRNA therapeutics differ in several critical and challenging ways.
A quick biotech lesson. If nucleic acids such as RNA are administered ‘naked’ into the body, they can be subject to rapid degradation by RNases (or ribonucleases) that are ubiquitous in the blood and tissues – rendering the administered RNA useless.
RNA exposed in the bloodstream can also generate adverse immune responses that also deactivate RNA and generate potentially dangerous adverse immune responses.
To solve these problems, nucleic acids are often-times encapsulated in some sort of nano-carrier.
In mRNA vaccines, mRNA for the viral protein is wrapped inside the synthetic lipid nanoparticle (LNP). When injected into the patient’s muscle, the LNP-mRNA substance causes some cells to produce the viral protein in situ.
The foreign viral protein then triggers the patient’s immune system, stimulating immune-signal amplification pathways that convert a small amount of exposed viral protein into a potent immune memory against future infection.
The future of gene therapy for other needs
Many medical problems could be solved with additive gene therapy, where the aim is to generate meaningful amounts of a gene-product that is lacking in that person due to aging, genetic disorders, or other causes.
A therapeutic mRNA product could become an additive gene therapy by delivering the required RNA sequence for the gene-product into target cells, so that the cell’s own protein-producing ribosomes transcribe the RNA in situ and produce the desired gene-product.
An example for such a therapy could be the delivery of mRNA that is encoding the CF transmembrane conductance regulator (CFTR) gene into lung cells to treat Cystic Fibrosis (CF) by having lung cells produce functional CFTR.
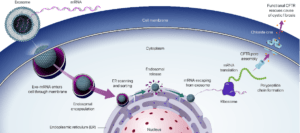
Using exosome therapeutic RNA to treat Cystic Fibrosis. Source: Exopharm
However, therapeutic mRNA products are different from mRNA vaccines.
Unlike mRNA vaccines which rely upon the patient’s immune system to amplify the effect of the viral protein produced in situ, for mRNA therapeutics the amount of gene-product generated in situ must reach biologically meaningful levels for a positive medical effect.
“The paper by Rohner et al says that mRNA therapeutics require as much as a 1,000-fold-higher level of in situ protein production compared with an RNA vaccine to reach a therapeutic threshold,” Dixon said.
What sort of nano-carrier is well-suited to mRNA therapeutic products?
Given that mRNA is best delivered in a nano-carrier, Dixon said the choices are limited and come down to lipid nanoparticles (LNPs) and exosomes – both nano-sized particles that can be loaded with mRNA.
So, what exactly are exosomes? They are best described as extracellular vesicles, natural nano-sized particles produced by cells to exchange materials and genetic instructions and coordinate cellular activities in our bodies.
Exosomes are released naturally by virtually every cell type.
“Studies have shown that targeted delivery is a major hurdle for effective RNA therapeutics, a hurdle that must be overcome to broaden the application of clinical translation of this type of therapeutic,” Dixon said.
“Exosomes can be designed and manufactured to target selected cells, tissues and organs.”
Solving challenges of mRNA therapeutics
mRNA therapeutics may require a patient to be dosed daily, weekly, or monthly to solve the deficiency being treated – so frequent repeat dosing will be a feature of many mRNA therapeutics.
There is concern that repeated dosing of therapeutic RNA may cause an unwanted or potentially dangerous immune response.
“Immune and cytotoxic responses to LNP-encapsulated mRNA have been a major concern in early clinical studies,” the paper by Rohner et al reports, based on research published in 2021.
Exosomes tested by Exopharm so far do not show signs of generating an adverse immune response in animal testing, even after 10 repeated doses.
Dixon said Chein and his fellow authors are experts in RNA therapeutics and have canvassed the delivery alternatives for these products.
“Their work highlights the potential advantages of exosomes over LNPs,” he said.
“Exosomes are nature’s RNA delivery vehicles – nano-scale particles released and received by cells around the body, acting as a biomolecule parcel delivery service.”
The paper by Rohner and colleagues, notes limitations of LNP drug-delivery technology including low biocompatibility, elevated immunogenicity, toxicity and cytotoxicity, limited tissue-tropism and poor persistence if administered systemically.
Whereas exosomes are described as having high biocompatibility, low immunogenicity, low tumorigenesis, providing tropism, penetrating blood-brain barrier and an endogenous (non-synthetic) delivery of cargo RNA.
Unlike LNPs, which were first developed over 30 years ago, exosomes are a recently emerging method for drug-delivery.
But the ability to manufacture exosomes as a large-scale drug-delivery chassis has required several manufacturing challenges to be solved.
Exopharm’s exosomes a game-changer in RNA therapeutics
Exopharm was established in 2013 with the express focus to develop all the technologies required to make exosomes a viable industrial-scale drug-delivery chassis.
It is the only listed exosome company on the ASX, and one of a small number of exosome companies listed globally.
Exopharm has demonstrated its 7 game-changing technologies and has answers to the challenges identified in the Unlocking the promise of mRNA therapeutics study.
“For each of the drug-delivery issues identified by Rohner et al, the exosomes from Exopharm’s technologies have an answer,” Dixon said.
“Exopharm and exosomes are now well-positioned to enable mRNA therapeutics live up to their promise.
“Exopharm and its exosomes can now contribute to the limitless future of RNA therapeutics.”
This article was developed in collaboration with Exopharm, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
You might be interested in













