You might be interested in
Health & Biotech
'Tide is turning for the healthcare sector' – ASX biotechs making strong gains in 2024 Part 2
Health & Biotech
The next Neuren? Nyrada chases after two big markets - stroke and traumatic brain injury
News
Neuren Pharmaceuticals may have secured an all-important deal to get its genetic brain disorder drug out of the lab and into the market.
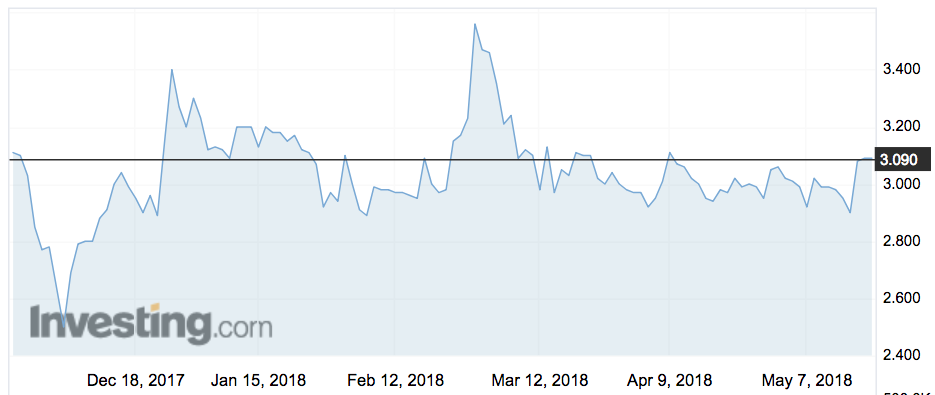
Its shares jumped 10 per cent on Monday morning to $3.40.
Neuren (ASX:NEU) is talking to an unnamed US pharmaceuticals business to commercialise a drug called trofinetide.
The US company has a three-month exclusivity period.
Trofinetide finished Phase 2 trials for Rett syndrome last year, a genetic disorder found only in women. In October Neuren announced it would do a Phase 3 trial for this syndrome.
The Phase 2 trial for Fragile X syndrome, another genetic disorder which causes intellectual disabilities, finished in 2015. It still needs to undergo a Phase 3 trial.
(Clinical trials are generally divided into three phases. Phase 1 focuses on safety, Phase 2 tests for effectiveness and Phase 3 examines whether the new drug is an improvement on existing treatment.)
Neuren’s treatment is an “orphan drug” — a classification for drugs that target rare diseases that encourages fast-track development and incentives to encourage companies to make them.
The US company is investing $US4 million ($5.3 million) for 1.3 million Neuren shares to pay for the exclusivity period.
That values Neuren at $4 a share. It closed on Friday at $3.09. The company has not traded above $3.60 in the last year.
A $US500,000 break fee is payable if either party breaks off the transaction.