Heart-stopping moments ahead for Uscom investors
Health & Biotech
Health & Biotech
Uscom — a maker of non-invasive cardiac and pulmonary measuring devices — deserves high praise indeed as it looks to the heavens for revenue growth from seven planned product releases this year.
We’re talking literally: Uscom’s (ASX:UCM) BP+ device to measure blood pressure is used on the International Space Station, where hypertension becomes an issue when the cosmonauts break out the vodka and start messing with the controls.
BP+ was also used to measure the blood pressure of 90 British service men and women scaling 5.3km up Mt Everest.
The study, published in the Journal of Human Hypertension, concluded the systolic BP measurement of the device was superior to the old brachial method of blowing up a cuff on the upper arm.
As far as we know, they all made it back with nary a yeti scratch.
The Sydney based, China-focused Uscom (as in Ultrasonic Cardiac Output Monitors) has forged a quiet but successful path in the global medical device sphere since listing in December 2003.
The technology was invented by ultrasound specialist and University of Queensland professor of medicine Prof Rob Phillips.
A Coffs Harbour native, Prof Phillips saw a better way of measuring blood flows in and around the heart than a highly invasive pulmonary artery catheter.
We’re sure some folk thought he was bananas, but Uscom has built to a $3.5 million a year turnover company with one approved product on market.
Prof Phillips is executive chairman with a 17 per cent stake. While the corporate governance rule book frowns on such arrangements, Phillips reckons that a principal with skin in the game is one of the best predictors of success.
Uscom has won the notable support of Chinese health entrepreneur Stephen Meng, who has recently bolstered his holding from 10 per cent to 16.88 per cent. This was through a placement at 13.5c a share, followed by on-market buying at 22c a pop.
Meng is the founder of the $30 billion Beijing-based, Hong Kong-listed Sihuan Pharma, so he is a key asset for Uscom for navigating China’s tricky corporate and regulatory culture.
Prof Phillips says Mr Meng has spent 20 years improving China sales and distribution for Sihuan, which now has 3000 distributors selling to 10,000 hospitals.
Uscom currently has around 40 global distributors. Given success in China is all about scale, one doesn’t have to be a Mensa graduate to nut out what this one’s all about.
Sweet product suite
Uscom currently has three products. The first, Uscom 1A, is a cardiac output monitor for sepsis, fluid, heart failure, hypertension and pre-eclampsia (hypertension in pregnancy).
BP+, a central blood pressure monitor, was owned by New Zealand’s Pulsecore, and bought by Uscom in 2013.
Acquired via the purchase of the Budapest based Thor Laboratories in 2015, Spirosonic is an ultrasonic spirometer for asthma and chronic obstructive pulmonary disease (COPD).
All three are approved in Europe (CE Mark) and by the Aussie Therapeutics Goods Administration, while Uscom 1A is also approved in China (CFDA) and cleared in the US (as a 510K device).
BP+ and Spirosonic await approval in China, while Spirosonic also awaits US approval.
Last week the British and Irish Hypertension Society approved BP+ for specialist use, although for some reason strung-out investors birched the stock by 7 per cent.
Uscom’s focus is heavily on China, with 60 per cent of sales derived from selling to the Middle Kingdom via two master distributorships.
This proportion is expected to increase once the BP+ and Spirosonic devices receive Chinese approval.
“Blood pressure measurement techniques have almost always got it wrong because they measure the wrong things using the wrong devices,” Prof Phillips says. “It’s about the circulation and blood pressure is only a part of that.
“Furthermore, blood pressure in the arm is less accurate and predictive than pressure at the heart, which we measure with BP+.”
In the case of pre-eclampsia — the biggest cause of maternal foetal mortality — Uscom 1A can bring forward detection to the fifth week of the pregnancy, rather than the 20th week.
In other words, enough time for the clinicians to do something about it.
Uscom’s seven new products slated for release this year are best seen as extensions to Uscom’s current devices.
“These new devices are all non-invasive and digital and so perfectly suited to the global e-health [electronic health] revolution,” Prof Phillips says.
“While many — Google, Microsoft, Apple, Tencent and Huawei are among those developing e-health platforms — few have the front-end sensors to measure directly and accurately cardiovascular and pulmonary function,” Prof Phillips says.
Cardio vascular and pulmonary ailments account for 75 per cent of deaths globally (although Alzheimer’s disease is rapidly gaining ground in the mortality stakes, as we noted recently).
Progress to date
Uscom turned over $864,000 in 2011-12 and $3.5 million in 2016-17, a growth rate of more than 300 per cent.
With the imminent release of the BP+ and Spirosonic devices globally, Uscom should be nudging $30 million by 2022.
Indeed, management has enunciated short, mid and long-term revenue targets of $10 million, $20 million and $50 million, respectively.
Uscom lost $1.8 million in 2016-17 and has recorded similar deficits over the past five years.
However, Prof Phillips said the debt-free company was “intermittently” cash flow positive in 2016-17.
The December quarter cash generation of $40,000 is encouraging. “But I would prefer to maintain revenue growth and be cash-flow neutral,” he says.
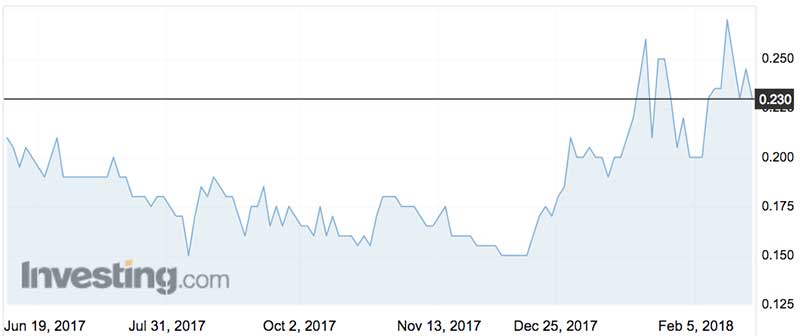
Share price blips after flat lining
In the last 12 months Uscom shares have traded between 15c (on August 9) and 26c (January 19).
The company listed in 2003 at $2 a share, which in hindsight was a tad steep given the shares have since traded as low as 6.7c (January 2012) recovering to a high of 85c (August 2009).
Recent good vibes — including Mr Meng’s buying — has seen the stock blip from 15c to 25c.
December quarter orders were for a record 68 units, up 24 per cent.
Prof Phillips and Mr Meng aside, Uscom is supported by a bevy of loyal investors including Gary Davey, who is better known in media as the current programming head of Rupert Murdoch’s Sky TV.
Prof Phillips and Mr Davey are old friends, with Mr Davey retiring as chief executive officer of Star in Hong Kong (temporarily as was the case) to Coffs Harbour.
Sheena Jack is a weighty addition to the cosy board, as her day job is as chief executive officer of one the country’s biggest health insurers, HCF.
(Shh! We won’t tell about the moonlighting if you don’t.)
Dr Boreham’s diagnosis
Uscom is hardly devoid of competitors: its own investor prezzo lists Uscom 1A as competing with 11 products made by seven manufacturers.
But only four are non-invasive. None, except for Uscom 1A, ticks all the boxes (once again, literally) on measures such as simplicity, cost and portability.
Uscom proves the adage that device development takes about five times longer than expected and is five times as hard.
But as Prof Phillips points out, Cochlear’s and Resmed’s intellectual property dates back to the 1970s.
Given the pending Chinese and US approvals, the next few months promise heart-stopping moments for investors.
This column first appeared in Biotech Daily
Disclosure: Dr Boreham is not a qualified medical practitioner, does not possess a doctorate of any sort and is yet to serve his term on the International Space Station.
The content of this article was not selected, modified or otherwise controlled by Stockhead. Stockhead has not provided, endorsed or otherwise assumed responsibility for any financial product advice contained in this article.