China hits pause on Eagle Health’s diet shake ads
Health & Biotech
Health & Biotech
Chinese authorities have stopped Eagle Health from marketing its much-promoted diet shake while it seeks the right registration.
The diet supplements seller (ASX:EHH) has been talking about the pre-meal diet shake since August last year and selling into China since December.
But regulators from the China Food and Drug Administration have put the brakes on.
The diet shake was being sold as a “functional food product” but marketed as a diabetes control aid.
Now the company is applying for “food for special medical purpose” registration and the regulator has stopped all ads implying it’s a medical product.
That is dampening sales, the company says.
In its half-year report issued two weeks ago, Eagle Health said a revenue bump of 16.4 per cent to $41.7 million was “mainly attributable” to a significant increase in marketing spending.
They are hoping for registration by the end of December.
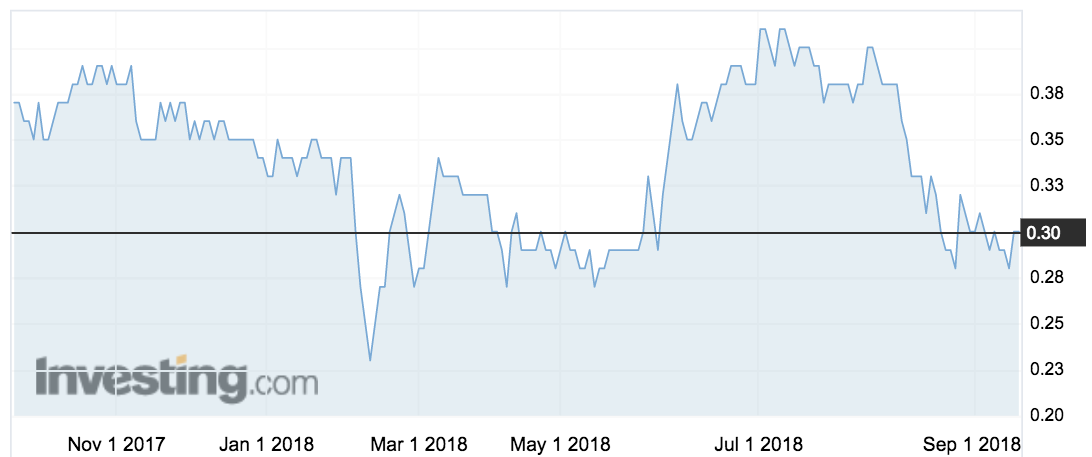
Eagle Health listed on the ASX in July 2017 after raising $25 million at 40c a pop. The stock is now trading at about 30c.
The company did say in its IPO prospectus that it expects medical registration in China to generally take 12 to 24 months.
Eagle Health’s 15-year licensing deal with the owner of the diet shake IP, Omni Innovation, is premised on CFDA registration.
When announcing the contract in August last year, two months after listing on the ASX, Eagle said $1 million of the licensing fee would be paid upfront and $500,000 paid after registration as a ‘medical food’ came through.
In its half-year report in August this year, Eagle Health listed that $500,000 as a contingent liability (a potential cost on the business).
Eagle Health also sells an ‘immortality herb’ in China, donkey-hide gelatin protein powder and yak bone powder, among other products.