You might be interested in
Health & Biotech
Health Kick Podcast: How Osteopore is innovating in the area of bone and tissue regeneration
Health & Biotech
Leading Aussie medtechs Osteopore and Singular Health reveal landmark deal
Health & Biotech
Health & Biotech
Special Report: Singapore-based Osteopore has won Australian regulatory approval for its bone healing facial and skull devices.
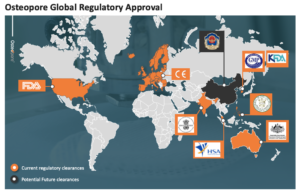
Osteopore (ASX:OSX) has added the Australian Therapeutics Goods Administration (TGA) to its ‘pool room shelf’ of regulators who think its bone healing products, in this case for the skull and face, are worthy of joining the Australian market.
The Singapore-based medical device company is commercialising a range of 3D printed bioresorbable scaffolds for regenerative bone healing, and in this case for use as bone “void” fillers.
The TGA has approved for use in Australia the Osteomesh, Osteoplug, and Osteoplug-C products, which European and US regulators have already done, together with Singapore and other East Asian regulatory clearances.
Osteomesh can be used in a range of cranial procedures, including reconstruction for orbital floor (the bone below the eye) fractures which can be caused when the area is hit with a blunt object.
The Osteoplug is used for neurosurgical burr holes and other cranial defects. A burr hole occurs which a piece of skull needs to be removed entirely, and is therefore can’t regenerate on its own.
The Osteoplug-C is also used for neurosurgical burr holes and other cranial defects in conjunction with cerebral shunt operations (a cerebral shunt is a device that removes fluid from around the brain and moves it to the abdomen, where it can be reabsorbed).
All three products have now been registered on the Australian Register of Therapeutic Goods (ARTG).
Osteopore’s craniofacial products will also be included in the Prosthesis Listing in July 2020.
“We are delighted to have now received TGA approval for Osteoplug and Osteomesh, and this has now opened up the Australian market to Osteopore. Australia is a key market for the global expansion of our business, and we are excited to be able to continue to build our revenue streams in this market,” says Osteopore CEO Goh Khoon Seng.
Obtaining TGA regulatory approval now allows Osteopore to make its products more broadly commercially available to doctors and hospitals across Australia, and the company will continue ongoing discussions with potential distribution partners.
Osteopore has an Australia-based business development consultant to lead its distribution strategy and drive sales.
These are not the first products Ostepore has had approved in Australia. Its scaffold technology has been used for customised implants under Special Access Scheme, where surgeons believe it to be the most applicable product to deliver the best patient outcomes.
Most notably, Osteopore’s technology was used in 2017 to enable the successful regeneration of a patient’s tibia after 36cm was removed due to infection, and this case received significant media coverage in late 2019 following the patient’s successful recovery.
Speaking with Stockhead, Osteopore Executive Director Geoff Pocock said “This is a significant step forward in Osteopore’s commercialisation. Securing TGA approval is a major step in establishing our Australian distribution and revenue streams.”