Another ASX health chief just said you’ve ‘misunderstood’ his announcement
Health & Biotech
Health & Biotech
The chief of biotech Mesoblast says investors “clearly misunderstood” results from the company’s heart failure trial after its shares dropped 28 per cent on Monday.
It’s the second time this month the boss of an ASX-listed health stock has blamed shareholders for misunderstanding an announcement.
Two weeks ago ResApp boss Tony Keating lamented that “we haven’t educated shareholders well enough on what a good result actually looks like” after his stock halved following what he described as “really positive” trial results.
On Monday Mesoblast boss Silviu Itescu told Stockhead he “could not be more excited about the outcome” of a trial that achieved “clinically meaningful” results in end-stage heart failure patients.
After the stock plummeted, Mr Itescu told Stockhead: “the announcement has clearly been misunderstood by the market.”
Mesoblast’s (ASX:MSB) Phase 2 trial results reported today showed its drug MPC-150-IM achieved a significant reduction in major gastrointestinal bleeding and hospitalisations in patients implanted with a left ventricular assist device (LVAD).
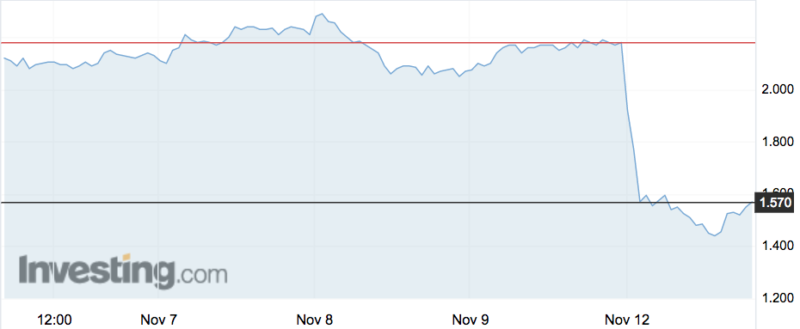
But investors sold down the stock after the report noted “the primary endpoint of temporary weaning from full LVAD support was not achieved overall”.
Mesoblast says US health authorities believed this was “not a clinically meaningful outcome in and of itself” — but that wasn’t enough for investors who rushed for the exit, pushing shares down to their lowest point since August:
A ‘primary endpoint’ of a clinical trial is the main result that is measured at the end of a study to see if a given treatment has worked.
Health and biotech investors look for this phrase in ASX announcements; as evidenced by Bionomics (ASX:BNO) shares diving when the primary endpoint of its PTSD trial was not met.
Mr Itescutold Stockhead: “For the primary endpoint in question, the FDA advised that it was a biomarker, not a clinically meaningful outcome in and of itself.
“There was a clinically meaningful reduction in hospitalisations and bleedings and that’s all that matters. We’re extremely excited about the outcome.
“I think the retail market does not fully appreciate the complex nature of the disease and the FDA endpoints and we need to educate them better. We need to make people more aware of the opportunity that is now in front of us thanks to these results.
“It provides a potential pathway to bring our heart failure product to market sooner for these patients in great need.”
Mesoblast’s main focus is making “mesenchymal lineage” adult stem cells to treat a wide range of conditions.
Mesenchymal stem cells are cells that can differentiate — the process by which a cell changes from one type to another — into many different cells, giving them great potential therapeutic benefit.