You might be interested in
Health & Biotech
ASX Health Stocks: Argenica flies over 15pc after FDA grants Rare Pediatric Disease Designation
News
Rise and Shine: Everything you need to know before the ASX opens
Health & Biotech
Health & Biotech
While the Greater Powers* did a pretty decent job with our main organs and brains and all that, they made shoddier work of the cartilage and tissues that hold us together.
The knee, in particular, must have come off Our Creator’s production line at 4.45pm on a Friday arvo.
Even the worst Kingswoods in the 1970s were better constructed than the anterior cruciate ligament (ACL), the tenuous strip of tissue that connects the thigh bone (femur) to the shin bone (tibia).
ACLs are the bane of sportspeople globally, with 100,000 to 200,000 ACL ruptures annually in the US alone.
You can also add 250,000 rotator cuff (shoulder) and 200,000 Achilles tendons to that market annually.
Perth-based regenerative medicine house Orthocell (ASX:OCC) is striving to rectify Our Creator’s shoddy work, as it develops and commercialises cell therapies and related technology to treat soft tissue injuries and musculo-skeletal disorders.
Orthocell was founded in 2006 by current chief executive officer Paul Anderson and chief scientific officer Prof Ming Hao Zheng, former chief executive officer of cell therapist Verigen (which the US-based Genzyme acquired in 2005).
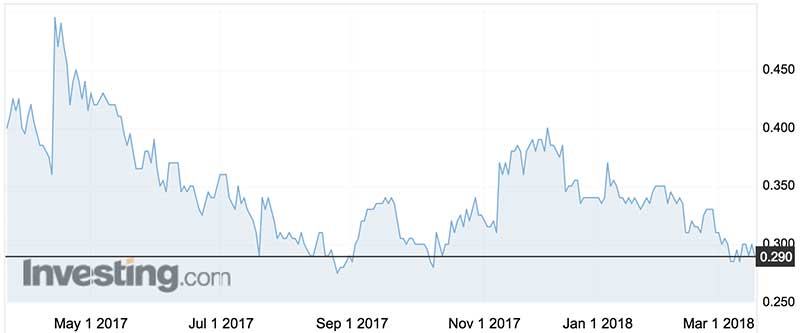
Having obtained earlier seed funding, Orthocell listed in August 2014 after raising $8 million at 40c a share.
“We are a mature company with lots of boxes ticked in lots of areas,” Mr Anderson says.
Orthocell’s products
Orthocell’s current products — autologous tendon implants (Ortho-ATI) and autologous chondocyte implants (Ortho-ACI) — involve novel uses of the patient’s own, that is autologous, tissue, namely tendon progenitor cells, to stimulate the growth of collagen and connective tissues.
Both products are approved for use in Australia, New Zealand, Singapore and Hong Kong under good manufacturing practice protocols.
Ortho-ATI is approved in the US, Europe, China and Japan and was said to be the first autologous cell therapy for tendon and ligament repair approved for sale in a major market.
The company’s product in development, Celgro, is a naturally-derived collagen for soft tissue repair. It is expected to have applications in tendons, peripheral nerve, bone and articular (joint) cartilage repair.
Celgro, has undergone pre-clinical evaluation as a collagen “rope” for ACL reconstructions.
The idea is that stem cells from the remains of the ACL grow into the rope and eventually integrate with the bone, creating a tensile strength similar to the natural ligament.
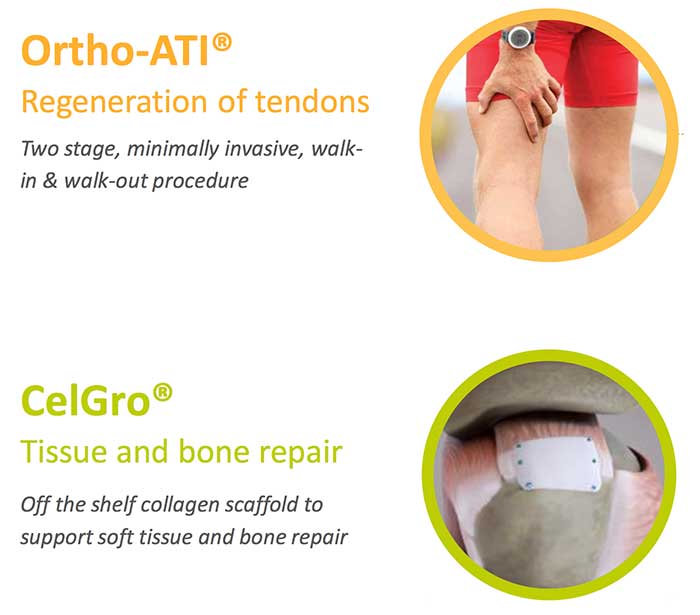
Ortho-ATI is for chronic tendon degeneration resistant to other therapies.
Clinical trials and case studies to date suggest the therapy hastens tendon healing and reduces pain and stiffness.
So far Ortho-ATI has been effective against gluteal tendinopathy (a common form of hip pain) and “extensor carpi radialis brevis”.
That’s tennis elbow to you and me.
Orthocell-ACI is for repair of articulating joint cartilages, mainly in the knee and ankle. It is hoped the procedure will be globally accepted as more effective and less painful than the traditional alternative of an autologous tendon graft.
So far, about 1000 patients have been treated with Ortho-ATI or Ortho-ACI — roughly evenly split between the therapies — in Australia, New Zealand, Singapore and Hong Kong.
In a key milestone, last November Orthocell obtained Conformité Européenne (CE) mark approval for use of Celgro for dental (bone) and facial (soft tissue) applications.
The company estimates the global dental market alone at $US600 million ($778 million) with 1.5 million procedures a year.
Unlike Orthocell’s other products, Celgro is regarded as a class-three-device rather than a cell therapy, because Celgro takes an animal raw material and processes it into various therapeutic tools.
This means a “simple and more linear” path to market.
“Celgro is a platform technology with multiple applications in multiple tissue types,” Mr Anderson says. “It is a big market with easier access to market.”
In the clinic
In early February, Orthocell said the top-line results of a preclinical ACL study of Celgro indicated “superior biomechanical properties”.
The study of 72 patients — half treated with Celgro and half with traditional autografts — showed that ligament stem cells from the ACL stump were capable of growing into the Celgro rope.
The purpose of the study, in conjunction with the University of Western Australia, was to show that the Celgro rope was equal or better than the use of autologous grafts from tissue typically harvested from hamstrings (which can result in the patient enjoying restored knees but then “doing a hammy”).
Mr Anderson says the data also supports Celgro’s potential as an off-the-shelf treatment across multiple indications including bone, tendon and peripheral nerves.
Celgro also won a good rap from Freddie Fu from the Pittsburgh School of Medicine.
Freddie Who?
The rock star of orthopaedic surgery, Prof Fu has been entrusted to treat the knees of high-profile athletes including Zlatan Ibrahimovic.
It must have worked, because the Manchester United striker arrived at training the other day in a GBP210,000 ($380,000) Porsche.
In the case of Ortho-ATI, recruiting is underway for a 30-patient, randomised trial aiming to replace corticosteroids to treat rotor cuff tendinopathy. This program is in collaboration with DePuy Synthes Products, an arm of Johnson & Johnson.
In December, Orthocell reported a case study of swimmer Christian Sprenger treated with Ortho-ATI for shoulder tendon pain, which enabled him to return to competition.
(By the way, most sportspeople don’t want to admit to dodgy knees and shoulders, especially at contract renegotiation time).
In September, the company reported the safety and efficacy of the first three patients treated with Celgro for hip joint cartilage degeneration. This one was in league with prominent orthopaedic surgeon Dr John O’Donnell, at Melbourne’s St Vincent’s Hospital.
Now for the revenue …
In January, Orthocell boosted its coffers with a $3 million placement and share purchase plan. The funds will be used to further US trials, accelerate the Celgro commercialization and advance development of Ortho-ATI.
Revenue has been modest to date, derived from local Ortho-ACI and Ortho-ATI sales.
Mr Anderson says the company does not have a sales force in Australia, so the product take-up to date should be seen more as validating the technology and the TGA-approved, Perth manufacturing facility.
“The fact we have treated 1,000 patients means we have been able to optimize our manufacturing process, which is incredibly important for those large international partners,” Mr Anderson says.
“Our approach is to validate locally but commercialise internationally. After all, there are more people in Texas than Australia.”
Orthocell shares legged it up to 92c in mid-2015, but in the last year they’ve traded in a range of 28c to 50c.
Dr Boreham’s diagnosis:
Like Cynata (ASX:CYP) which we covered recently, Orthocell’s valuation is only a fraction of that of stem-cell leader Mesoblast (ASX:MSB).
Locally, Orthocell is perhaps better compared with Admedus (ASX: AHZ), which has developed scaffold products for heart and vascular repair.
As usual there’s a flattering Nasdaq comparison: Vericel, which has one ACL product and bears a $US400 million market cap.
In October last year Smith & Nephew acquired the private medical scaffold outfit Rotation Medical for up to $US210 million.
Orthocell is not for weak-kneed investors. Crucible would like to see more revenue through the door and, of course, more regulatory breakthroughs including US approval.
The company is due to start selling Celgro in Europe this month, but meaningful sales will take time to build.
Orthocell is discussing commercialising Celgro with a European partner and is having similar deep and meaningful with parties in the US, Japan and here.
Given the recent global takeover activity in the biotech sector, we’ll class Orthocell as a possible target.
* Your columnist is ecumenical and could be talking about Mother Nature.
This column first appeared in Biotech Daily
Disclosure: Dr Boreham is not a qualified medical practitioner and does not possess a doctorate of any sort. But he does possess two knees that still perform the task His Creator intended.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions. The content of this article was not selected, modified or otherwise controlled by Stockhead. Stockhead has not provided, endorsed or otherwise assumed responsibility for any financial product advice contained in this article.