Why Botanix could be the cannabis tortise that won the race
Health & Biotech
Health & Biotech
In the second of a three-part series on ASX cannabis stocks, top biotech columnist Tim Boreham profiles Botanix Pharmaceuticals
Are investors who have weighed into the ASX-listed medical (or quasi-medical) pot plays a pack of dopes?
After all, the world’s most powerful medical regulator has made his views crystal clear on the medical cannabis caper, warning that the only dinky-di route to market is via properly structured clinical trials.
“Advancing sound development programs that properly evaluate active ingredients contained in medical marijuana can lead to important medical therapies,” intoned US Food and Drug Administration commissioner Dr Scott Gottlieb, MD.
“Controlled clinical trials, along with careful review through the FDA’s drug approval process are the most appropriate way to bring marijuana-derived treatments to patients.”
Dr Gottlieb also warned the regulator would take action when it sees “illegal marketing of CBD [cannabidiol] products with unproven medical claims”.
The good doc was commenting after the US FDA green-lighted the epilepsy drug Epidiolex, the first cannabis treatment to be approved by the regulator.
Botanix’s zit-busting quest
Both the approval of Epidiolex and Dr Gottlieb’s firm stance are music to the ears of Botanix, which is one of the few ASX listed pot stocks to have a kosher clinical program for its two synthetic marijuana-based compounds.
In fact, Botanix highlighted Dr Gottlieb’s words in its own material.
Botanix is working on topical treatments for skin conditions such as acne, psoriasis and atopic dermatitis (severe eczema).
The dreaded zits are a key target, given that 250 million people globally and 50 million Americans (mainly teenagers) are blighted by acne.
Botanix also has an exclusive licence to a device called Permetrex, a dermal delivery device allowing 10 to 20 times more of the active ingredient to get through the skin.
Cannabis has been crushed and used on the skin for thousands of years. “We know it works, but not why,” says Botanix chief executive officer Matt Callahan.
One barrier to understanding is that as only humans get acne, animal models aren’t available.
Mr Callahan says cannabis has three mechanisms of action: it stops the sebaceous oils, reduces inflammation and kills bacteria.
Interestingly, Botanix’s material does not refer to the ‘c’ cannabis word but a “synthetic form of a widely-studied natural compound”.
As Mr Callahan argues, aspirin (acetylsalicylic acid, if you really need to know) derives from the bark of a willow tree but no-one calls it a tree drug.
“The cannabis plant is the inspiration. We are just using a chemical that happens to be found in the plant.”
Having said that, this column is the second of our three-part series on so-called ‘pot stocks’, so sorry about that, bud.
From old Bone to new hound on the block
Botanix listed in July 2016 in a back-door raising using the shelf of Perth-based biotech Bone Medical. The company raised $3.5 million at two cents apiece in the process.
Headquartered in Perth but based in Philadelphia, Botanix was founded by parties including Mr Callahan, Caperi (a financial investor) and scientist Robert Bosch.
The short and the long of Bone was that it was established by Dr Roger New’s Oxford UK-based Proxima to develop Capthymone for osteoporosis and BN006 for rheumatoid arthritis, licenced from Proxima.
The trials showed the drugs didn’t work, Bone went into a spiral dive with a La Jolla Cove equity draw-down facility paying significant fees to Proxima.
Cornerstone’s Robert Towner replaced Dr New as chairman and went looking for something to backdoor.
Botanix wanted somewhere to list and voila! – a listing without a technology for a technology without a listing.
Very cute
Mr Callahan is founding CEO of Iceutica Inc and Churchill Pharma, having co-invented Iceutica’s drug delivery platform.
Iceutica was due to list in 2011, but instead the backers sold the company for two times the expected listing value.
“It’s the most successful company no-one has ever heard of,” he says.
A lawyer, Mr Callahan has also been involved with two venture capital firms and is a director of Orthocell.
Botanix chairman Graham Griffiths headed a listed Perth-based outfit called Ipernica, which initially focused on intellectual property but spawned a successful listed aerial mapping offshoot called Nearmap.
The Permetrex technology was developed by a dude called Dr Eugene Cooper, who has licensed its use to Botanix. Dr Cooper also developed another drug delivery mechanism, Nano Crystal, which is used in six FDA approved products.
News from the clinic
In January, Botanix finished its initial trial of BTX1503 for acne. In June it recruited the first of 360 patients for a phase II trial to be completed by mid-2019.
The enrolled patients will have moderate to severe acne and will be treated for 12 weeks at dermatological clinics here and in the US.
Botanix also finished a phase 1b study for atopic dermatitis in June and is preparing for a phase 2 trial.
A further phase 1b study for BTX1308 targeting psoriasis is due to start this month.
(Ed: Clinical trials are generally divided into three phases. Phase 1 focuses on safety, Phase 2 tests for effectiveness and Phase 3 examines whether the new drug is an improvement on existing treatment. Sometimes trials are further divided into parts A and B, where a B stage is generally more rigorous.)
In the case of the phase 1 pimple trial, Botanix earlier this year reported that of the 21 subjects with moderate to severe acne, 18 completed the program.
On average, inflammatory lesions (papules and pustules, if you really want to know) declined by an average 47 percent after 28 days of treatment.
This compares with reductions of 42 per cent and 38 per cent respectively for the two leading existing acne treatments: Allergan’s Epiduo and Aczone’s Galderma.
Financials and performance
Botanix is nicely cashed up after raising $23 million in two placements, both at 11c apiece. Both were oversubscribed.
The phase 2 studies are costed at $6 million to $8 million (each or combined) and are described by management as fully-funded.
With $17 million in the bank and a $3.5 million Federal R&D Tax Incentive in the offing, there’s more than enough of the other green stuff to cover the earlier-stage work and management has no plans to raise more.
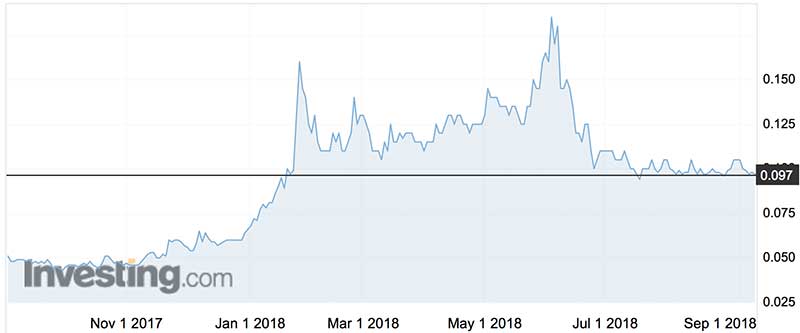
Botanix shares peaked at 18.5c on May 31, having troughed at 4.3c on October 4 last year.
Dr Boreham’s diagnosis
Mr Callahan says the acne market globally is worth $US5 billion and dermatitis is worth $US7 billion.
“What’s interesting about these markets is there has been little innovation over the last 20 years,” he says. “With acne, there has been no new approved drug for over 20 years.”
An approved drug called Accutane (or Roaccutane) is the only one to reduce sebaceous oil levels, the underlying cause of acne.
But the drug’s side effects include lymphoma, birth defects and suicide risks, which makes putting up with a blemished visage that little more acceptable.
It’s no wonder the generic versions, which remain on market after Roche pulled its branded product, bear a stern FDA ‘black box’ warning.
Atopic dermatitis is usually treated with steroids, but in the case of children there’s the risk of stunting growth with prolonged use.
“Our goal is to develop a product with significant efficacy but without the baggage of many of the products in the market,” Mr Callahan says.
He says Botanix could have gone the over-the-counter route, competing with non-approved CBD creams that sell for $US50 to $US100 a tube.
But by gaining Dr Gottlieb’s seal of approval, Botanix is more likely to be able to sell its unguents for $US2,000 to $US5,000 a tube in the US market (if reimbursable).
GW Pharma, the owner of Epidiolex, last month revealed the cost of the drug would be around $US32,500 a year.
Mr Callahan is heartened that big pharma Pfizer in 2016 bought Anacor — which was in phase 3 dermatitis trials – for $US5 billion.
Botanix’s serious clinical approach means a slower and costlier path to market than, say, developing an over-the-counter zit treatment.
But the eventual returns are higher as well.
Given that, Botanix’s $75 million market cap — modest for the pot sector, which of course Botanix isn’t a part — means the company looks a decent punt.
Other pot stocks having pot shots at skin disorders are the Europe-based MGC Pharmaceuticals (ASX:MXC) and Zelda Therapeutics (ASX:ZLD), which is carrying out Chilean based eczema trials.
This column first appeared in Biotech Daily
Disclosure: Dr Boreham is not a qualified medical practitioner and does not possess a doctorate of any sort. Happily, teenage pimples only caused him a spot of bother.
The content of this article was not selected, modified or otherwise controlled by Stockhead. Stockhead has not provided, endorsed or otherwise assumed responsibility for any financial product advice contained in this article.