You might be interested in
Health & Biotech
ASX Health Stocks: Healius rips higher as new CEO takes over; Nova Eye bags record US sales
Health & Biotech
ASX Health Stocks: Recce, Cynata say their diabetic foot ulcer trials are going great guns
News
Health & Biotech
Special report: Treatment for steroid-resistant, acute graft-versus-host disease (GvHD) — which has mortality rates in excess of 90 per cent — could be a step closer as Australian stem cell and regenerative medicine company Cynata Therapeutics approaches dosing of the final patient in its Phase I trial.
GvHD is a medical complication that can occur after a bone marrow transplant.
The condition is now treated with steroids. But in many cases this is unsuccessful and leaves no alternative for patients.
Under the Cynata (ASX:CYP) trial, which began a year ago, up to 16 patients are treated with CYP-001 — Cynata’s first mesenchymal stem cell (MSC) product.
The patients are split into two cohorts. Those in the second cohort (Cohort B) receive double the dose of CYP-001 compared with patients in the first cohort (Cohort A).
The dosing of the final patient in Cohort B will mark the completion of enrolment in the clinical trial, which is the world’s first stem cell clinical trial to use an MSC product derived from an induced pluripotent stem cell (iPSC) sourced from a single blood donation from one donor.
An iPSC is an adult stem cell that has been made by reprogramming a blood cell back into an embryonic-like state and giving it the ability to develop into an essentially unlimited amount of other cells.
It is Cynata’s proprietary CymerusTM platform that enables it to manufacture an unlimited source of MSCs from iPSCs, creating a consistent, scalable and therapeutic grade stem cell product.
The trial’s primary objective is to assess the safety and tolerability of CYP-001, alongside an evaluation of the efficacy of the treatment.
Once all patients are dosed the next steps are the data analysis of the safety and efficacy of CYP-001 at the 28-day and 100-day points.
Full data expected in 2018
The data analysis will be released by Cynata once all enrolled patients reach these milestones. The full data set is expected by the fourth quarter of 2018.
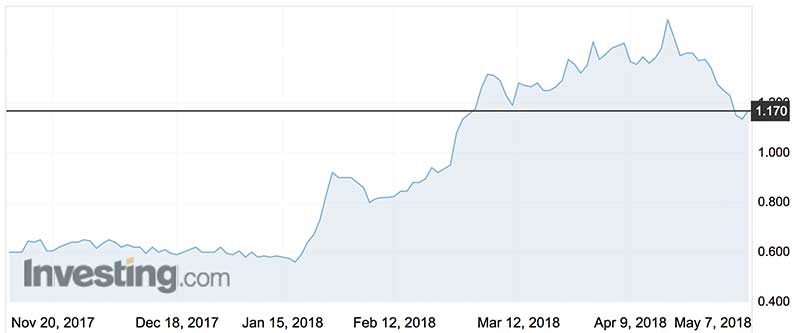
Interim safety and efficacy data from Cohort A, released to the ASX on January 22, was positive and well-received by the market.
The shares are now well over $1 — up from 59c beforethe Interim Analysis results.
This has also piqued the interest of global fund manager Fidelity International, which has been buying stock on market since the end of January and now hold a 5 per cent stake.
The analysis showed all eight patients demonstrated at least a ‘Partial Response’ — defined as an improvement in the severity of their condition compared to baseline.
One patient later died from an unrelated pneumonia affliction, a common occurrence in recipients of bone marrow transplants and similar procedures and deemed to be unrelated to the trial by the independent Data Safety and Monitoring Board.
Further efficacy data at the 100-day mark for Cohort A showed that, in addition to the Partial Response, four out of eight patients showed a ‘Complete Response’ – that being all signs and symptoms of their GvHD were completely resolved.
Dr Ross Macdonald, Cynata’s CEO, said “the completion of enrolment for Cohort B will be an important step towards a treatment for patients.
“GvHD is a devastating and incurable disease, and there is a huge unmet need for new therapies to treat this often-fatal affliction.
“The potential success of this trial would put Cynata one step closer to addressing this need and we’re looking forward to the analysis of the full data set.”
Full completion of the trial will trigger the expiry period of the licence option agreement with Japanese industrial giant FUJIFILM, which can be exercised anytime up until 90 days after the trial results are received.
The licence option agreement is worth a total of USD3m in upfront fees and a further $60 million in milestone payments, plus double-digit royalties.
FUJIFILM is also a major shareholder in Cynata, with a 9 per cent stake in the company following a $4 million investment in 2017.
Cynata is also focused on further commercialising its CymerusTM platform in other indications and is undertaking preclinical and proof-of-concept studies continue in areas including asthma, heart attack, brain cancer and acute respiratory distress syndrome.
This special report is brought to you by Cynata Therapeutics.
This advice has been prepared without taking into account your objectives, financial situation or needs. You should, therefore, consider the appropriateness of the advice, in light of your own objectives, financial situation or needs, before acting on the advice.
If this advice relates to the acquisition, or possible acquisition, of a particular financial product, the recipient should obtain a disclosure document, a Product Disclosure Statement or an offer document (PDS) relating to the product and consider the PDS before making any decision about whether to acquire the product.