REVA shares surge after passing Go, collecting 7 new countries to sell stents into
Health & Biotech
Health & Biotech
REVA Medical shares shot up after it reported penetration into seven new countries.
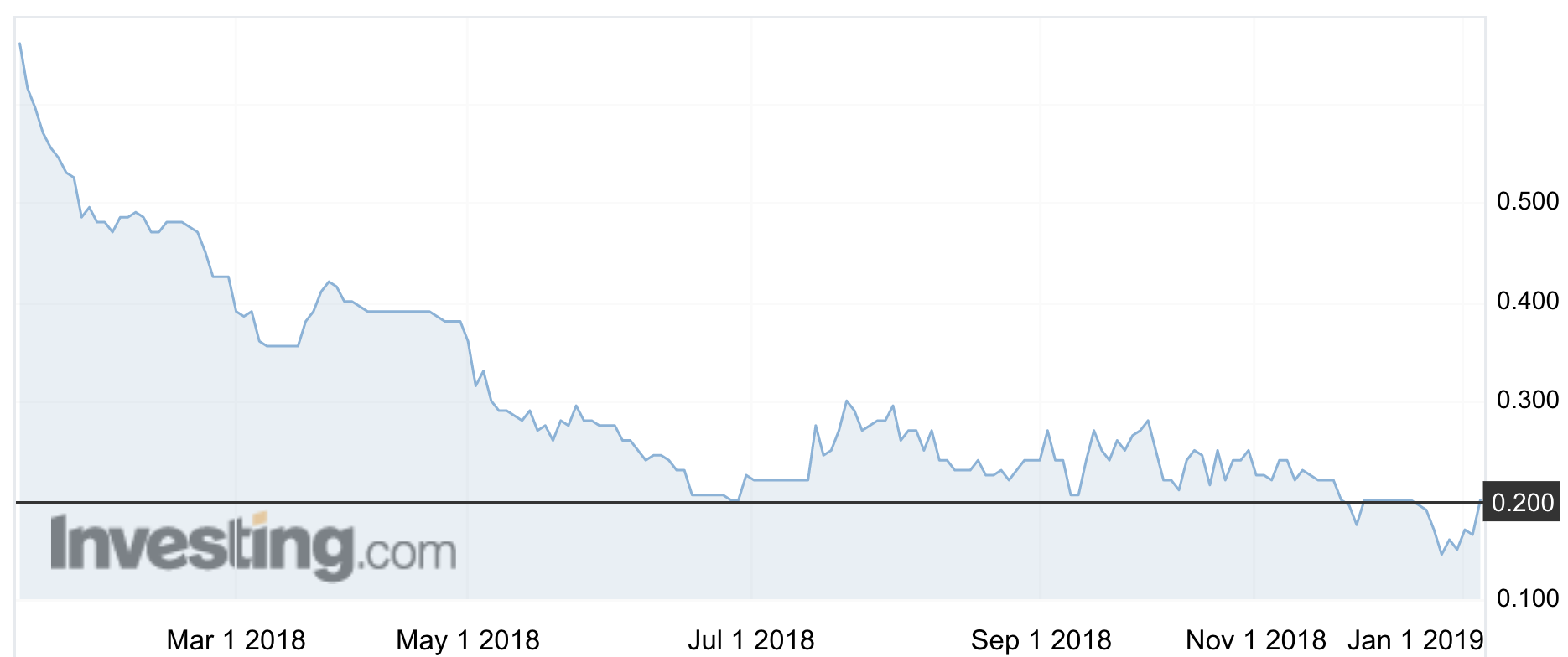
The stock rose 21 per cent on Monday morning to 20c, but is still well off the highs of 2016 that saw it reach $1.30.
The company’s (ASX:RVA) shares have been on a continuous downward slide since October 2016.
On Monday it said it had secured commercial distribution deals in six Eastern Europe countries and Russia for the Fantom Encore bio-scaffold.
A tubular sleeve in a lattice formation, Fantom is made out of advanced polymers and is thinner than rival stents with “enhanced deliverability while being visible under X-ray”.
The scaffold is drug eluting, which means it can house and slowly release a drug to prevent the growth of cells causing the artery blockage.
The absorption allows for restoration of the ‘natural movement’ of the artery.
The company says the seven countries represent a $290 million medical device market, with 350,000 surgeries performed in that specific space annually.
REVA started selling in June last year in Germany, Switzerland and Austria, and its country list now includes Italy and Turkey as well.
Critically however, REVA does not have approval from the US Food and Drug Administration and is yet to apply, with no stated timeline for doing so.
It received a CE mark for the Fantom device in April 2017, allowing it to sell in Europe, and said at the start of 2018 they were working towards getting conditional US Investigational Device Exemption approval to conduct an FDA clinical trial by the end of 2018.
REVA has been contacted to confirm whether or not that approval came through.